When it comes to sample preparation techniques there are a ton of techniques and acronyms to keep track of. These include solid phase extract (SPE), supported liquid extraction (SLE); liquid-liquid extraction (LLE); solid phase microextraction (SPME); and salt-assisted liquid-liquid extraction (SALLE), just to name a few. There are many things to consider when choosing a type of sample preparation technique, such as analyte properties, price of consumables, and downstream sensitivity of samples. This blog will primarily focus on salt-assisted liquid-liquid extraction (SALLE).
Salt-assisted liquid-liquid extraction (SALLE), also known as “salting out extraction,” is a subset of liquid-liquid extraction. It may also be referred to as “salt-induced separation,” or “salting-out homogenous liquid-liquid extraction” (SHLLE). [1] In SALLE, a high concentration of salt is added to the aqueous phase. Unlike LLE, it is typical to use water and a water-miscible organic solvent, such as acetonitrile, ethanol, or isopropyl alcohol, when performing SALLE. While water is miscible with these solvents, the addition of the salt creates a phase separation, which allows for a liquid-liquid extraction to take place. A schematic of this extraction procedure can be found in Figure 1 below.
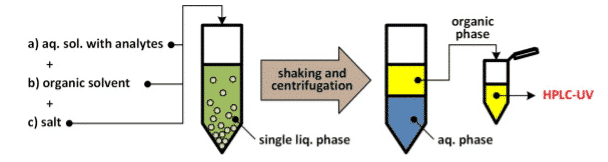
SALLE is very similar to another popular sample preparation technique called QuEChERS. QuEChERS stands for Quick, Easy, Cheap, Effective, Rugged, and Safe. This technique involves an initial partitioning using salt, normally available in easy-to-use packets that can be added to the samples. This step works under the same principle as SALLE, where the salts help to create the phase separation. This is a very popular technique for the analysis of pesticides in foods, like fruits and vegetables, but has been successfully applied to other markets as well. The main difference between SALLE and QuEChERS is that QuEChERS usually involves a dSPE, or dispersive solid phase extraction, step for additional sample cleanup, where SALLE does not include this step.
While traditional LLE does not work well with polar analytes, the addition of the salt in SALLE enhances the ability of the extraction to work with polar analytes. This makes it ideal for testing for drugs in biological matrices, which can vary in their degrees of polarity. It also works for a broader group of compounds than some other methods, like SLE, that need to be optimized based on specific analyte properties. In a recent application note, sample preparation techniques were compared for the analysis of drugs of abuse in oral fluids. In this work, the SALLE method worked for a broad range of 68 analytes, including benzodiazepines, opioids, stimulants, and novel psychoactive substances. Oral fluid samples often contain surfactants from the buffer in the kits. These surfactants can lead to issues downstream with matrix effects and column degradation. A sample cleanup step is necessary to remove these surfactants, but it is important to find one in this case that works for a wide range of analytes with different properties. SALLE was the perfect choice for this application because of its efficient cleanup and ability to work with polar analytes and analytes from different classes of drugs. The full application note can be found here.
Another benefit to SALLE is that it often requires less solvent than typical LLE workflows, making it safer to work with and resulting in less waste. As more and more methods are moving towards greener solutions, limited solvent and waste is a benefit to using this extraction method. SALLE is also more easily amenable to automation as compared to some typical LLE methods because of the smaller volumes required for SALLE.
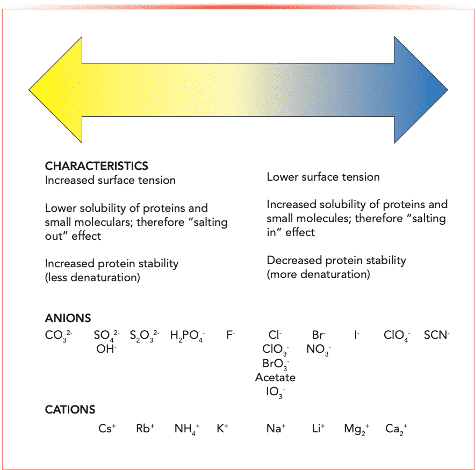
There are a few important variables to consider when using SALLE as a sample preparation technique. One of these parameters is the type of salt that is used in the extraction, as different salts have different extraction capabilities. The most common salt choices are calcium chloride, sodium chloride, and magnesium sulfate. [3] Another variable to be considered is the concentration of the salt solution, because different salts at different concentrations result in varying degrees of phase separation. [3] It is understood that anions undergo salting out more readily than cations. [2] An example of what ions are more likely to participate in salting out are detailed by something called the Hofmeister series, which can be found in Figure 2 below, in which the ions are from most to least participating from the left to right respectively.
If you are looking for a simple but effective sample clean up for a broad list of analytes, SALLE may be a good option. Additionally, if you are looking to move towards greener solutions, SALLE boasts some benefits in that category as well. Like all parts of method development, choosing a sample preparation technique that works for all analytes can take a bit of trial and error. Understanding the mechanisms of these extraction techniques as well as analyte properties is a vital part in selecting the right technique.
References and Further Reading
1. I.M. Valente, et. al., Another glimpse over the salting-out assisted liquid-liquid extraction in acetonitrile/water mixtures, J. Chromatogr. A., 1308 (2013) 58-62. https://doi.org/10.1016/j.chroma.2013.08.014
2. D.E. Raynie, Enhancing extractions by salting out, LC GC N. Am., 41 (7) (2023) 262-265. https://doi.org/10.56530/lcgc.na.ax9490j4
3. R.E. Majors, Salting-out liquid-liquid extraction (SALLE), LCGC Int., 27 (7)( 2009) 526-533. https://www.chromatographyonline.com/view/salting-out-liquid-liquid-extraction-salle


