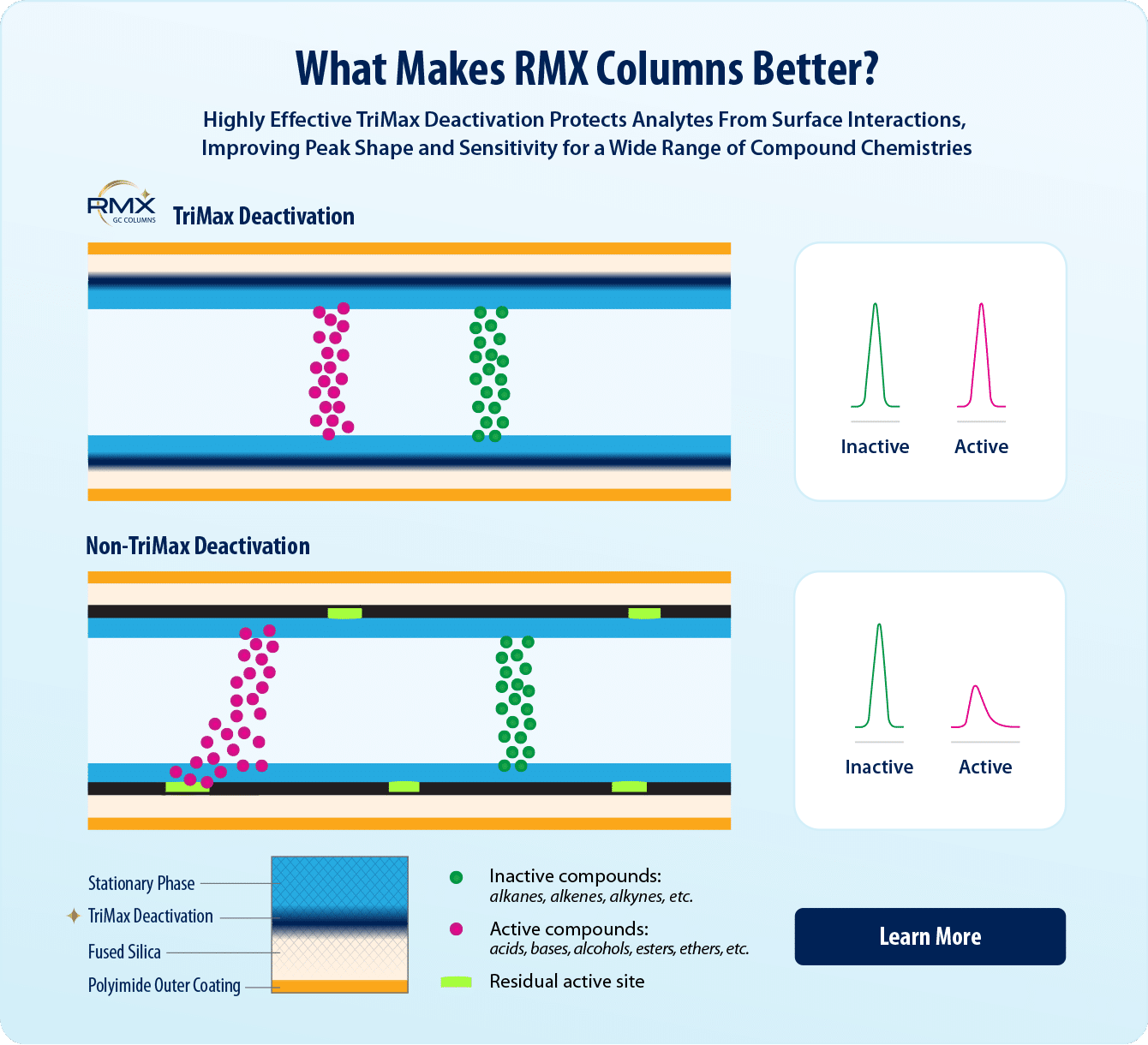
- Leverage next-generation TriMax column deactivation to lower detection limits and still meet data quality requirements.
- Exceptionally inert sample flow path ensures sharp, symmetrical peaks for a wide range of challenging semivolatiles.
- Established GC-MS conditions for both a robust scan method and a sensitive SIM method.


Environmental laboratories analyzing semivolatiles are under continual pressure to achieve lower detection limits due to customer demands or the need to reduce solvent use for either regulatory reasons or to reduce costs and improve profitability. MS-based methods, such as EPA Method 8270, are a common approach for analysis, and offer scientists the flexibility of choosing instrumentation to achieve the lowest possible limits of detection via MS. Some labs may opt for sensitive instrumentation, such as GC-MS/MS, or use GC-MS with selected ion monitoring (SIM), while others may opt for more traditional scan methods. SIM and scan methods can typically be performed on the same instrument, where scan methods are simple and versatile, collecting signals for a range of compound masses, while SIM allows for greater sensitivity for specific ions in specific retention time windows. SIM methods are typically more complicated to set up and maintain, but as labs explore strategies to lower detection limits, GC-MS in SIM mode may be considered as an alternative to GC-MS/MS without labs having to budget for a new instrument.
In this study, we established conditions for GC-MS semivolatiles analysis under both scan and SIM modes to provide labs with both a simple, robust scan workflow as well as a highly sensitive SIM alternative to GC-MS/MS (Figure 1). An RMX-5Sil MS column was selected for both methods because it provides standard 5sil selectivity with a next-generation TriMax deactivation that has been proven to provide an exceptionally inert sample flow path [1,2]. As shown in Figure 1, peak asymmetry consistently met quality objectives (≤2) for key analytes (benzidine, pentachlorophenol, and 2,4-dinitrophenol). In addition, resolution criteria (>50%) were easily met for challenging PAHs with the valley between benzo[b]fluoranthene and benzo[k]fluoranthene >94%, and the valley between indeno[1,2,3-cd]pyrene, and dibenz[a,h]anthracene >95%. Using an RMX-5Sil MS column under the conditions shown here, excellent results were obtained for a wide range of semivolatiles at very low levels (1 ng on-column in scan mode and 200 pg on-column in SIM mode), allowing labs to work confidently at the lower end of MS detection limits.
Scan Mode (1 ng on-column)
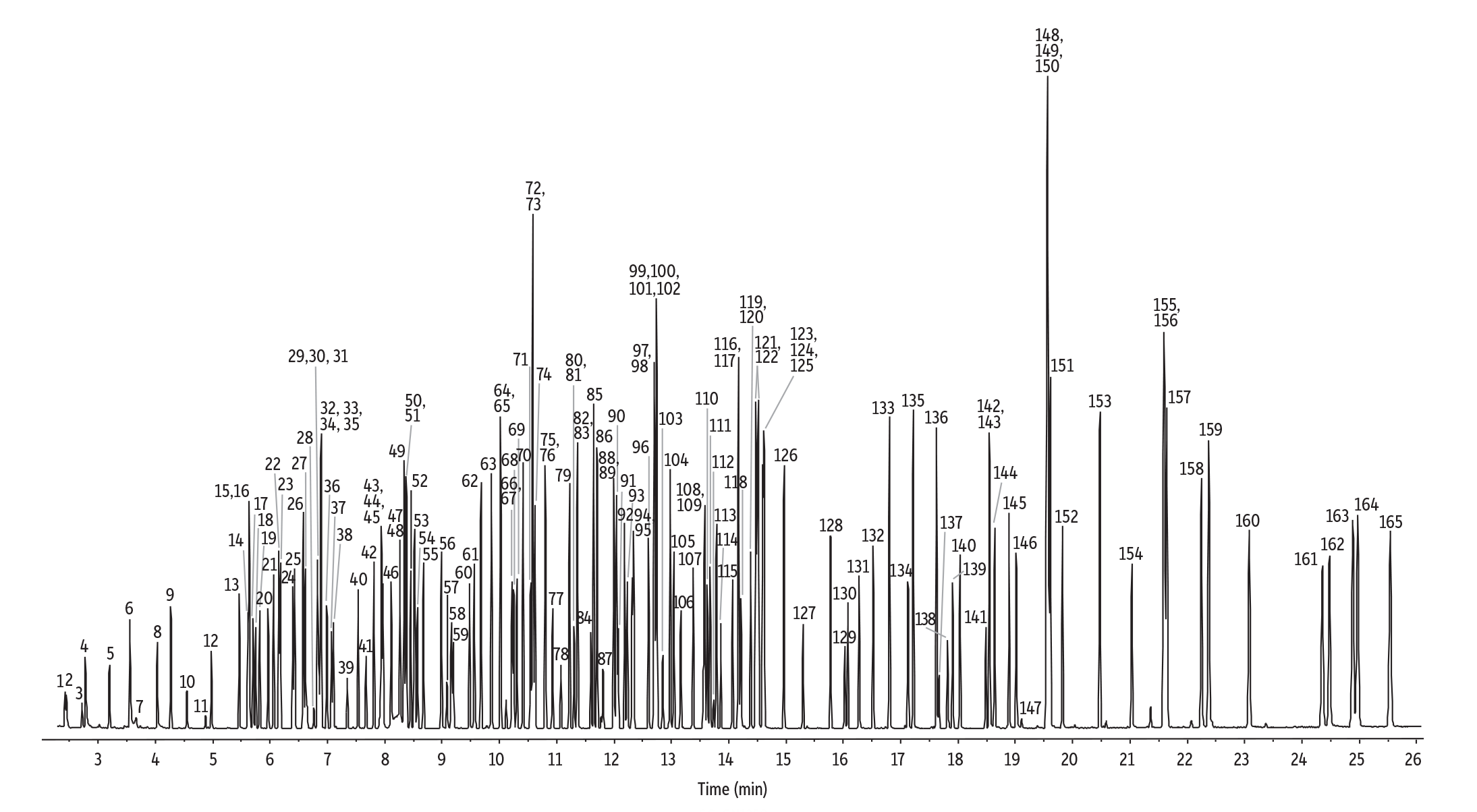
GC_GN1250
Peaks
Conditions
| Column | RMX-5Sil MS, 30 m, 0.25 mm ID, 0.25 µm (cat.# 17323) |
|---|---|
| Standard/Sample | |
| Methapyrilene (cat.# 32460) | |
| Appendix IX mix #1, revised (cat.# 32459) | |
| SVOC additions (cat.# 31909) | |
| Benzoic acid (cat.# 31879) | |
| 8270 MegaMix (cat.# 31850) | |
| Appendix IX mix #2 (cat.# 31806) | |
| Acid surrogate mix (4/89 SOW) (cat.# 31025) | |
| Base neutral surrogate mix (4/89 SOW) (cat.# 31024) | |
| Revised SV internal standard mix (cat.# 31886) | |
| Diluent: | Methylene chloride |
| Conc.: | 1 µg/mL |
| Injection | |
| Inj. Vol.: | 1 µL split (split ratio 10:1) |
| Liner: | Topaz 4.0 mm ID single taper liner w/wool (cat.# 23303) |
| Inj. Temp.: | 250 °C |
| Split Vent Flow Rate: | 12 mL/min |
| Oven | |
| Oven Temp.: | 40 °C (hold 1 min) to 280 °C at 12.4 °C/min to 330 °C at 3.3 °C/min (hold 3.65 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 1.2 mL/min |
| Linear Velocity: | 39.7 cm/sec |
| Detector | MS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mode: | Scan | ||||||||
| Scan Program: | |||||||||
| |||||||||
| Transfer Line Temp.: | 280 °C | ||||||||
| Analyzer Type: | Quadrupole | ||||||||
| Source Temp.: | 330 °C | ||||||||
| Quad Temp.: | 180 °C | ||||||||
| Solvent Delay Time: | 2 min | ||||||||
| Tune Type: | PFTBA | ||||||||
| Ionization Mode: | EI | ||||||||
| Instrument | Agilent 7890B GC & 5977B MSD | ||||||||
| Notes | To simplify ordering, the SVOC MegaMix 150 kit (cat.# 31907) contains one ampul each of the following standards that were used in this experiment. • 8270 MegaMix (cat.# 31850) • SVOC Additions standard (cat.# 31909) • Appendix IX mix #1, revised (cat.# 32459) • Methapyrilene (cat.# 32460) • Appendix IX mix #2 (cat.# 31806) • Benzoic acid (cat.# 31879) | ||||||||
SIM Mode (200 pg on-column)
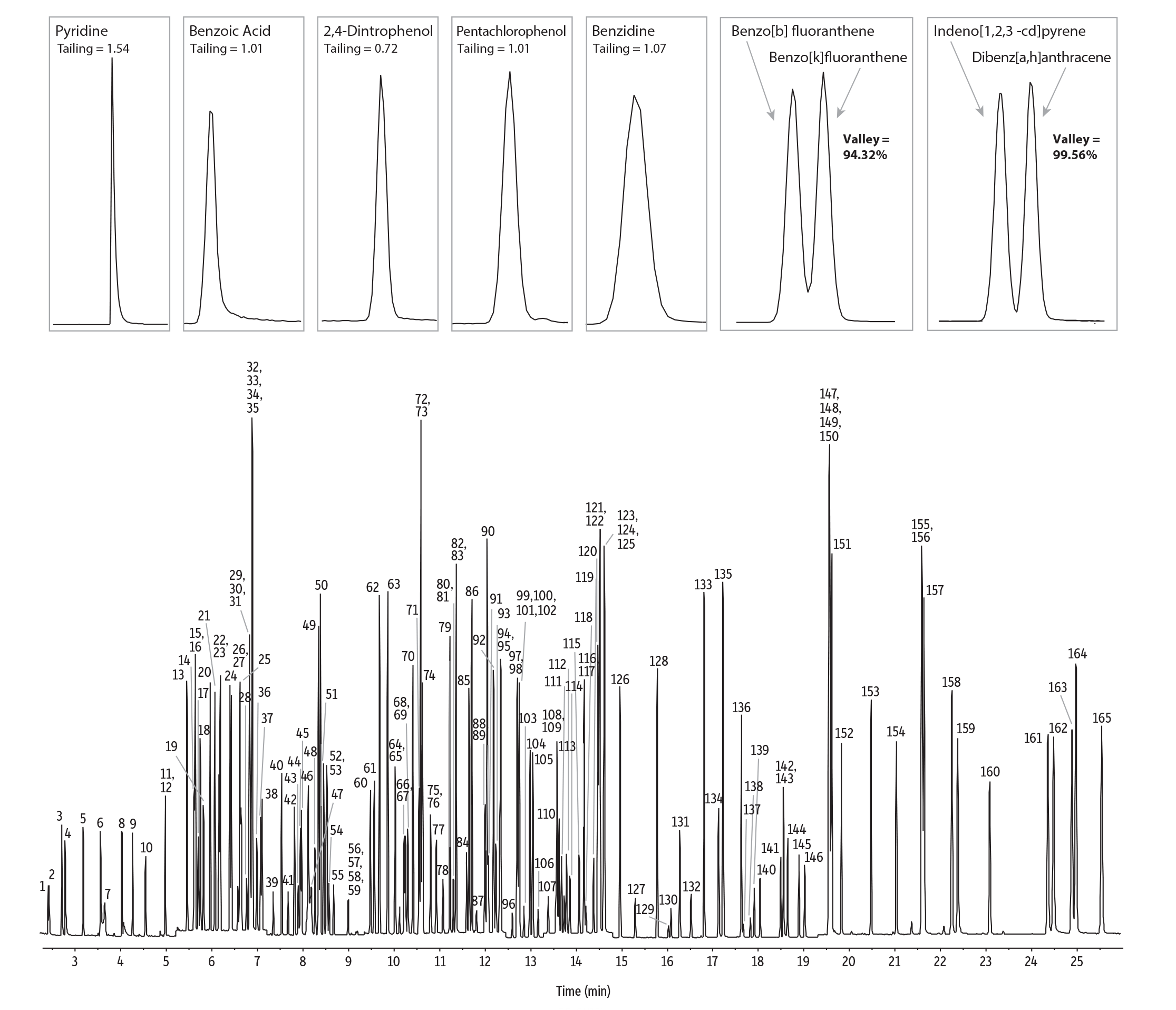
GC_GN1251
Peaks
| Peaks | tR (min) | Conc. (ng/mL) | SIM Ion | Dwell Time (ms) | Ion Group | |
|---|---|---|---|---|---|---|
| 1. | 1,4-Dioxane-d8 | 2.401 | 200 | 96 | 25 | 1 |
| 2. | 1,4-Dioxane | 2.427 | 200 | 88 | 25 | 1 |
| 3. | N-Nitrosodimethylamine | 2.702 | 200 | 74 | 25 | 1 |
| 4. | Pyridine | 2.774 | 200 | 79 | 25 | 1 |
| 5. | Ethyl methacrylate | 3.175 | 200 | 69 | 25 | 1 |
| 6. | 2-Picoline | 3.554 | 200 | 93 | 25 | 1 |
| 7. | N-Nitrosomethylethylamine | 3.648 | 200 | 42 | 25 | 1 |
| 8. | Methyl methanesulfonate | 4.021 | 200 | 80 | 25 | 1 |
| 9. | 2-Fluorophenol | 4.258 | 200 | 112 | 25 | 1 |
| 10. | Acrylamide | 4.543 | 200 | 71 | 25 | 1 |
| 11. | N-Nitrosodiethylamine | 4.977 | 200 | 102 | 25 | 1 |
| 12. | Ethyl methanesulfonate | 4.977 | 200 | 109 | 25 | 1 |
| 13. | Benzaldehyde | 5.454 | 200 | 77 | 11 | 2 |
| 14. | Phenol-d6 | 5.612 | 200 | 99 | 11 | 2 |
| 15. | Phenol | 5.633 | 200 | 94 | 11 | 2 |
| 16. | Aniline | 5.633 | 200 | 93 | 11 | 2 |
| 17. | Pentachloroethane | 5.7 | 200 | 167 | 11 | 2 |
| 18. | Bis(2-chloroethyl)ether | 5.743 | 200 | 63 | 11 | 2 |
| 19. | 2-Chlorophenol | 5.814 | 200 | 128 | 11 | 2 |
| 20. | n-Decane (C10) | 5.962 | 200 | 57 | 11 | 2 |
| 21. | 1,3-Dichlorobenzene | 6.066 | 200 | 146 | 11 | 2 |
| 22. | 1,4-Dichlorobenzene-d4 | 6.154 | 200 | 150 | 11 | 2 |
| 23. | 1,4-Dichlorobenzene | 6.18 | 200 | 148 | 11 | 2 |
| 24. | Benzyl alcohol | 6.393 | 200 | 79 | 11 | 2 |
| 25. | 1,2-Dichlorobenzene | 6.426 | 200 | 111 | 11 | 2 |
| 26. | 2-Methylphenol (o-cresol) | 6.611 | 200 | 108 | 11 | 2 |
| 27. | 2,2′-Oxybis(1-chloropropane) | 6.634 | 200 | 45 | 11 | 2 |
| 28. | N-Nitrosopyrrolidine | 6.765 | 200 | 100 | 11 | 2 |
| 29. | Acetophenone | 6.825 | 200 | 105 | 11 | 2 |
| 30. | N-Nitrosomorpholine | 6.825 | 200 | 56 | 11 | 2 |
| 31. | N-Nitroso-di-n-propylamine | 6.825 | 200 | 43 | 11 | 2 |
| 32. | 3-Methylphenol (m-cresol) | 6.885 | 200 | 80 | 11 | 2 |
| 33. | 4-Methylphenol (p-cresol) | 6.885 | 200 | 107 | 11 | 2 |
| 34. | Indene | 6.885 | 200 | 117 | 11 | 2 |
| 35. | o-Toluidine | 6.885 | 200 | 106 | 11 | 2 |
| 36. | Hexachloroethane | 6.989 | 200 | 119 | 11 | 2 |
| 37. | Nitrobenzene-d5 | 7.068 | 200 | 82 | 11 | 2 |
| 38. | Nitrobenzene | 7.1 | 200 | 123 | 11 | 2 |
| 39. | N-Nitrosopiperidine | 7.347 | 200 | 114 | 17 | 3 |
| 40. | Isophorone | 7.531 | 200 | 82 | 17 | 3 |
| 41. | 2-Nitrophenol | 7.671 | 200 | 139 | 17 | 3 |
| 42. | 2,4-Dimethylphenol | 7.811 | 200 | 122 | 17 | 3 |
| 43. | Benzoic acid | 7.898 | 200 | 105 | 17 | 3 |
| 44. | O,O,O-Triethyl phosphorothioate | 7.942 | 200 | 121 | 17 | 3 |
| 45. | Bis(2-chloroethoxy)methane | 7.969 | 200 | 93 | 17 | 3 |
| 46. | 2,4-Dichlorophenol | 8.113 | 200 | 162 | 17 | 3 |
| 47. | α,α-Dimethylphenethylamine (phentermine) | 8.178 | 200 | 58 | 17 | 3 |
| 48. | 1,2,4-Trichlorobenzene | 8.264 | 200 | 180 | 17 | 3 |
| 49. | Naphthalene-d8 | 8.34 | 200 | 136 | 17 | 3 |
| 50. | Naphthalene | 8.378 | 200 | 128 | 17 | 3 |
| 51. | α-Terpineol | 8.453 | 200 | 59 | 17 | 3 |
| 52. | 4-Chloroaniline | 8.518 | 200 | 127 | 17 | 3 |
| 53. | 2,6-Dichlorophenol | 8.518 | 200 | 164 | 17 | 3 |
| 54. | Hexachloropropene | 8.572 | 200 | 213 | 17 | 3 |
| 55. | Hexachlorobutadiene | 8.674 | 200 | 225 | 17 | 3 |
| 56. | Quinoline | 8.993 | 200 | 129 | 15 | 4 |
| 57. | ε-Caprolactam | 9.085 | 200 | 55 | 15 | 4 |
| 58. | 1,4-Phenylenediamine | 9.160 | 200 | 108 | 15 | 4 |
| 59. | N-Nitrosodibutylamine | 9.195 | 200 | 84 | 15 | 4 |
| 60. | 4-Chloro-3-methylphenol | 9.477 | 200 | 107 | 15 | 4 |
| 61. | Isosafrole isomer 2 | 9.563 | 200 | 181 | 15 | 4 |
| 62. | 2-Methylnaphthalene | 9.681 | 200 | 142 | 15 | 4 |
| 63. | 1-Methylnaphthalene | 9.858 | 200 | 141 | 15 | 4 |
| 64. | 1,2,4,5-Tetrachlorobenzene | 10.024 | 200 | 216 | 15 | 4 |
| 65. | Hexachlorocyclopentadiene | 10.024 | 200 | 237 | 15 | 4 |
| 66. | 2,3-Dichloroaniline | 10.221 | 200 | 161 | 15 | 4 |
| 67. | Isosafrole isomer 1 | 10.221 | 200 | 104 | 15 | 4 |
| 68. | 2,4,6-Trichlorophenol | 10.25 | 200 | 196 | 15 | 4 |
| 69. | 2,4,5-Trichlorophenol | 10.303 | 200 | 198 | 15 | 4 |
| 70. | 2-Fluorobiphenyl | 10.41 | 200 | 172 | 15 | 4 |
| 71. | Safrole | 10.549 | 200 | 131 | 15 | 4 |
| 72. | Biphenyl | 10.586 | 200 | 154 | 15 | 4 |
| 73. | 2-Chloronaphthalene | 10.586 | 200 | 162 | 15 | 4 |
| 74. | 1-Chloronaphthalene | 10.624 | 200 | 127 | 15 | 4 |
| 75. | 2-Nitroaniline | 10.803 | 200 | 138 | 13 | 5 |
| 76. | Diphenyl ether | 10.803 | 200 | 170 | 15 | 5 |
| 77. | 1,4-Naphthoquinone | 10.927 | 200 | 158 | 13 | 5 |
| 78. | 1,4-Dinitrobenzene | 11.073 | 200 | 168 | 13 | 5 |
| 79. | Dimethylphthalate | 11.223 | 200 | 163 | 13 | 5 |
| 80. | 1,3-Dinitrobenzene | 11.301 | 200 | 76 | 13 | 5 |
| 81. | 2,6-Dinitrotoluene | 11.301 | 200 | 165 | 13 | 5 |
| 82. | 1,2-Dinitrobenzene | 11.363 | 200 | 50 | 13 | 5 |
| Peaks | tR (min) | Conc. (ng/mL) | SIM Ion | Dwell Time (ms) | Ion Group | |
|---|---|---|---|---|---|---|
| 83. | Acenaphthylene | 11.363 | 200 | 152 | 13 | 5 |
| 84. | 3-Nitroaniline | 11.591 | 200 | 65 | 13 | 5 |
| 85. | Acenaphthene-d10 | 11.649 | 200 | 162 | 13 | 5 |
| 86. | Acenaphthene | 11.706 | 200 | 153 | 13 | 5 |
| 87. | 2,4-Dinitrophenol | 11.809 | 200 | 184 | 13 | 5 |
| 88. | 4-Nitrophenol | 11.996 | 200 | 139 | 13 | 5 |
| 89. | Pentachlorobenzene | 11.996 | 200 | 250 | 13 | 5 |
| 90. | Dibenzofuran | 12.043 | 200 | 84 | 13 | 5 |
| 91. | 2,4-Dinitrotoluene | 12.183 | 200 | 89 | 13 | 5 |
| 92. | 1-Naphthylamine (1-aminonaphthalene) | 12.235 | 200 | 143 | 13 | 5 |
| 93. | 2,3,5,6-Tetrachlorophenol | 12.339 | 200 | 232 | 13 | 5 |
| 94. | 2,3,4,6-Tetrachlorophenol | 12.339 | 200 | 230 | 13 | 5 |
| 95. | 2-Naphthylamine (2-aminonaphthalene) | 12.339 | 200 | 115 | 13 | 5 |
| 96. | Diethylphthalate | 12.596 | 200 | 149 | 13 | 5 |
| 97. | n-Hexadecane (C16) | 12.703 | 200 | 57 | 29 | 6 |
| 98. | Fluorene | 12.703 | 200 | 166 | 29 | 6 |
| 99. | Zalophus (thionazine) | 12.746 | 200 | 107 | 29 | 6 |
| 100. | 4-Chlorophenyl phenyl ether | 12.746 | 200 | 204 | 29 | 6 |
| 101. | 4-Nitroaniline | 12.746 | 200 | 65 | 29 | 6 |
| 102. | 5-Nitro-o-toluidine | 12.746 | 200 | 152 | 29 | 6 |
| 103. | 4,6-Dinitro-2-methylphenol (Dinitro-o-cresol) | 12.852 | 200 | 198 | 29 | 6 |
| 104. | Diphenylamine† | 12.98 | 200 | 169 | 29 | 6 |
| 105. | Azobenzene* | 13.044 | 200 | 77 | 29 | 6 |
| 106. | 2,4,6-Tribromophenol | 13.162 | 200 | 330 | 29 | 6 |
| 107. | Sulfotepp | 13.385 | 200 | 322 | 14 | 7 |
| 108. | 1,3,5-Trinitrobenzene | 13.581 | 200 | 213 | 14 | 7 |
| 109. | Phenacetin | 13.581 | 200 | 108 | 14 | 7 |
| 110. | Phorate | 13.618 | 200 | 75 | 14 | 7 |
| 111. | 4-Bromophenyl phenyl ether | 13.677 | 200 | 248 | 14 | 7 |
| 112. | Diallate | 13.735 | 200 | 43 | 14 | 7 |
| 113. | Hexachlorobenzene | 13.788 | 200 | 284 | 14 | 7 |
| 114. | Dimethoate | 13.857 | 200 | 87 | 14 | 7 |
| 115. | Atrazine | 14.064 | 200 | 200 | 14 | 7 |
| 116. | 4-Aminobiphenyl | 14.17 | 200 | 169 | 14 | 7 |
| 117. | Pentachlorophenol | 14.17 | 200 | 266 | 14 | 7 |
| 118. | Pentachloronitrobenzene (Quintozene) | 14.213 | 200 | 237 | 14 | 7 |
| 119. | Propyzamide | 14.377 | 200 | 173 | 14 | 7 |
| 120. | Phenanthrene-d10 | 14.477 | 200 | 188 | 14 | 7 |
| 121. | n-Octadecane (C18) | 14.52 | 200 | 57 | 14 | 7 |
| 122. | Phenanthrene | 14.52 | 200 | 178 | 14 | 7 |
| 123. | Dinoseb | 14.616 | 200 | 211 | 14 | 7 |
| 124. | Disulfoton | 14.616 | 200 | 88 | 14 | 7 |
| 125. | Anthracene | 14.616 | 200 | 179 | 14 | 7 |
| 126. | Carbazole | 14.961 | 200 | 167 | 30 | 8 |
| 127. | Methyl parathion | 15.296 | 200 | 109 | 30 | 8 |
| 128. | Di-n-butyl phthalate | 15.779 | 200 | 149 | 30 | 8 |
| 129. | 4-Nitroquinoline-N-oxide | 16.026 | 200 | 190 | 30 | 8 |
| 130. | Parathion (ethyl parathion) | 16.075 | 200 | 291 | 30 | 8 |
| 131. | Methapyrilene hydrochloride | 16.273 | 200 | 58 | 30 | 8 |
| 132. | Isodrin | 16.52 | 200 | 193 | 30 | 8 |
| 133. | Fluoranthene | 16.806 | 200 | 202 | 30 | 8 |
| 134. | Benzidine | 17.124 | 200 | 184 | 30 | 8 |
| 135. | Pyrene | 17.223 | 200 | 203 | 30 | 8 |
| 136. | p-Terphenyl-d14 | 17.627 | 200 | 244 | 27 | 9 |
| 137. | Aramite isomer 2 | 17.627 | 200 | 175 | 27 | 9 |
| 138. | Aramite isomer 1 | 17.818 | 200 | 135 | 27 | 9 |
| 139. | p-Dimethylaminoazobenzene | 17.905 | 200 | 120 | 27 | 9 |
| 140. | Chlorobenzilate | 18.041 | 200 | 251 | 27 | 9 |
| 141. | Famphur | 18.487 | 200 | 218 | 27 | 9 |
| 142. | 3,3′-Dimethylbenzidine (o-tolidine) | 18.553 | 200 | 254 | 15 | 9 |
| 143. | Kepone | 18.553 | 200 | 272 | 27 | 9 |
| 144. | Benzyl butyl phthalate | 18.64 | 200 | 149 | 27 | 9 |
| 145. | Bis(2-ethylhexyl)adipate | 18.89 | 200 | 129 | 27 | 9 |
| 146. | 2-Acetylaminofluorene | 19.015 | 200 | 181 | 27 | 9 |
| 147. | 4,4′-Methylene-bis(2-chloroaniline) | 19.561 | 200 | 231 | 15 | 9 |
| 148. | 3,3′-Dichlorobenzidine | 19.561 | 200 | 212 | 27 | 9 |
| 149. | Benz[a]anthracene | 19.561 | 200 | 228 | 15 | 10 |
| 150. | Chrysene-d12 | 19.561 | 200 | 240 | 15 | 10 |
| 151. | Chrysene | 19.61 | 200 | 226 | 15 | 10 |
| 152. | Bis(2-ethylhexyl)phthalate | 19.824 | 200 | 167 | 15 | 10 |
| 153. | 6-Methylchrysene | 20.473 | 200 | 242 | 15 | 10 |
| 154. | Di-n-octyl phthalate | 21.031 | 200 | 149 | 15 | 10 |
| 155. | Benzo[b]fluoranthene | 21.589 | 200 | 57 | 15 | 10 |
| 156. | 7,12-Dimethylbenz[a]anthracene | 21.589 | 200 | 256 | 15 | 10 |
| 157. | Benzo[k]fluoranthene | 21.637 | 200 | 252 | 15 | 10 |
| 158. | Benzo[a]pyrene | 22.249 | 200 | 253 | 15 | 10 |
| 159. | Perylene-d12 | 22.377 | 200 | 264 | 15 | 10 |
| 160. | 3-Methylcholanthrene | 23.08 | 200 | 268 | 15 | 10 |
| 161. | Dibenz(a,h)acridine | 24.357 | 200 | 279 | 15 | 10 |
| 162. | Dibenz[a,j]acridine | 24.48 | 200 | 280 | 15 | 10 |
| 163. | Indeno[1,2,3-cd]pyrene | 24.882 | 200 | 277 | 15 | 10 |
| 164. | Dibenz[a,h]anthracene | 24.968 | 200 | 278 | 15 | 10 |
| 165. | Benzo[g,h,i]perylene | 25.537 | 200 | 276 | 15 | 10 |
Conditions
| Column | RMX-5Sil MS, 30 m, 0.25 mm ID, 0.25 µm (cat.# 17323) |
|---|---|
| Standard/Sample | |
| Methapyrilene (cat.# 32460) | |
| Appendix IX mix #1, revised (cat.# 32459) | |
| SVOC additions (cat.# 31909) | |
| Benzoic acid (cat.# 31879) | |
| 8270 MegaMix (cat.# 31850) | |
| Appendix IX mix #2 (cat.# 31806) | |
| Acid surrogate mix (4/89 SOW) (cat.# 31025) | |
| Base neutral surrogate mix (4/89 SOW) (cat.# 31024) | |
| Revised SV internal standard mix (cat.# 31886) | |
| Diluent: | Methylene chloride |
| Conc.: | 200 ng/mL |
| Injection | |
| Inj. Vol.: | 1 µL split (split ratio 10:1) |
| Liner: | Topaz 4.0 mm ID single taper liner w/wool (cat.# 23303) |
| Inj. Temp.: | 250 °C |
| Split Vent Flow Rate: | 12 mL/min |
| Oven | |
| Oven Temp.: | 40 °C (hold 1 min) to 280 °C at 12.4 °C/min to 330 °C at 3.3 °C/min (hold 3.65 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 1.2 mL/min |
| Linear Velocity: | 39.7 cm/sec |
| Detector | MS | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mode: | SIM | ||||||||||||||||||||||||||||||||||||||||||||
| SIM Program: | |||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||
| Transfer Line Temp.: | 280 °C | ||||||||||||||||||||||||||||||||||||||||||||
| Analyzer Type: | Quadrupole | ||||||||||||||||||||||||||||||||||||||||||||
| Source Temp.: | 330 °C | ||||||||||||||||||||||||||||||||||||||||||||
| Quad Temp.: | 180 °C | ||||||||||||||||||||||||||||||||||||||||||||
| Solvent Delay Time: | 2 min | ||||||||||||||||||||||||||||||||||||||||||||
| Tune Type: | PFTBA | ||||||||||||||||||||||||||||||||||||||||||||
| Ionization Mode: | EI | ||||||||||||||||||||||||||||||||||||||||||||
| Instrument | Agilent 7890B GC & 5977B MSD | ||||||||||||||||||||||||||||||||||||||||||||
| Notes | To simplify ordering, the SVOC MegaMix 150 kit (cat.# 31907) contains one ampul each of the following standards that were used in this experiment. • 8270 MegaMix (cat.# 31850) • SVOC Additions standard (cat.# 31909) • Appendix IX mix #1, revised (cat.# 32459) • Methapyrilene (cat.# 32460) • Appendix IX mix #2 (cat.# 31806) • Benzoic acid (cat.# 31879) | ||||||||||||||||||||||||||||||||||||||||||||
References
- E. Pack, J. Hoisington, C. English, R. Dhandapani, and C. Myers, Comprehensive trace-level semivolatiles analysis by GC-MS/MS (EPA Method 8270E), Application note, EVAN4919-US, Restek Corporation, 2025. https://discover.restek.com/application-notes/evan4919/comprehensive-trace-level-gc-ms-ms-semivolatiles-method-epa-method-8270e
- RMX GC columns brochure, GNBR4923-UNV, Restek Corporation, 2026. https://discover.restek.com/articles/gnbr4923/rmx-gc-columns-unleash-your-performance