Abstract
Testing for drugs of abuse in biological matrices is an important part of toxicology and workplace drug testing. The “gold standard” matrices that have been used for decades are typically blood and urine; however, the collection of these two matrices is invasive. Due to the ease of collection, oral fluid testing for drugs of abuse has been gaining popularity. However, this matrix comes with its own set of challenges not only related to the oral fluid itself but also due to the buffer used in collection devices, which contains preservatives and surfactants. Lengthy solid phase extraction (SPE) procedures are often used to remove these additives, so establishing a workflow that uses a simpler sample preparation workflow paired with accurate and robust quantitation is beneficial for laboratories running these tests. The primary objective of this work is to compare three sample preparation techniques: salt-assisted liquid-liquid extraction (SALLE); supported liquid extraction (SLE); and dilute-and-shoot for the analysis of drugs of abuse and novel psychoactive substances in oral fluid by LC-MS/MS.
Introduction
Testing for drugs of abuse in blood or urine is routine for many laboratories. These matrices have been used for decades, but oral fluid testing has been gaining popularity in recent years [1]. Oral fluid is a combination of saliva and everything else that is present in the mouth, including bacteria and food particles [2]. There has been an increase in its use because collection is easy and relatively noninvasive compared to other matrices [1]. Analyte levels in oral fluid also show good correlation with blood concentrations, and there is little potential for adulteration, dilution, or substitution, which can be problematic when using urine [1,2]. However, when using oral fluid, sample volume can be limited, and there are more variables that can affect the matrix, such as eating, drinking, or smoking before collection [1].
Sample preparation for oral fluid analysis usually involves SPE or a dilute-and-shoot approach. While oral fluids themselves are a relatively easy matrix to work with, the additives that are present in the buffer solutions in the collection kits can be problematic. Though these buffer solutions are necessary in order to preserve the sample and inhibit microbial growth, the additives can cause matrix effects and shorten the lifetime of the analytical column. SPE is more effective than dilute-and-shoot in mitigating the effects of the buffer additives, but the multi-step procedures typically used for drugs of abuse in oral fluids can be fairly complex and time-consuming.
When it comes to sample preparation, it is important to choose an efficient but effective approach. In this work, supported liquid extraction (SLE) and salt-assisted liquid-liquid extraction (SALLE) sample preparation techniques were performed and compared to a simple dilute-and-shoot approach. While sensitivity was too poor for dilute-and-shoot samples to warrant further assessment, SLE and SALLE samples produced good responses and were evaluated for accuracy, precision, and linearity.
Experimental
Calibration and QC Standards
A 10,000 ng/mL parent stock standard was created and then further diluted with methanol to prepare calibration standard solutions. The concentrations were as follows: 10,000, 5000, 2500, 1000, 500,100, 50, and 20 ng/mL. QC standards were prepared in a similar manner at 50, 650, 1500, and 6500 ng/mL. An internal standard was prepared at 100 ng/mL using the analytes listed in Table I.
To create the matrix-based calibrators and QC samples for testing, 100 µL of each standard was added to 900 µL of synthetic oral fluid (SMx oral fluid) (UTAK, Valencia, CA), creating a 1:10 dilution. Calibrators and QC samples were then processed according to the following sample preparation procedures.
Table I: Internal Standards and MRM Transitions
| Internal Standard | Precursor Ion | Product Ion 1 |
|---|---|---|
| Methamphetamine-D5 | 155.6 | 92.1 |
| Norfentanyl-D5 | 238.6 | 55.2 |
| Oxazepam-D5 | 292.3 | 246.1 |
| LSD-D3 | 327.2 | 179.9 |
| MDMA-D5 | 199.1 | 107.1 |
| Ketamine-D4 | 242.5 | 129.1 |
| 7-Aminoclonazepam-D4 | 290.4 | 121.0 |
| 6-Acetylmorphine-D3 | 331.3 | 165.0 |
| PCP-D5 | 249.4 | 96.2 |
| Fentanyl-D5 | 342.2 | 105.0 |
| EDDP-D3 | 280.7 | 234.2 |
| Hydromorphone-D3 | 289.1 | 184.9 |
| Methadone-D3 | 313.1 | 105.1 |
| Buprenorphine-D4 | 471.9 | 243.2 |
| Temazepam-D5 | 306.3 | 260.0 |
| Norbuprenorphine-D4 | 417.6 | 186.9 |
| Morphine-D3 | 289.6 | 164.9 |
| Oxycodone-D6 | 322.4 | 247.1 |
| Oxymorphone-D3 | 305.4 | 230.1 |
| Nordiazepam-D5 | 276.3 | 140.1 |
| Alprazolam-D5 | 314.2 | 286.0 |
| Isotonitazene-D7 | 418.2 | 100.0 |
| Cocaine-D3 | 307.3 | 185.3 |
| Lidocaine-D10 | 245.2 | 64.0 |
| delta-9-THC-D3 | 318.2 | 193.1 |
| Gabapentin-D10 | 182.0 | 147.2 |
Sample Preparation Techniques
Dilute-and-Shoot
For the dilute-and-shoot samples, 1 mL of prepared calibrator or QC sample was added to 3 mL of Quantisal buffer (Immunalysis, Pomona, CA) to simulate real-world ratios. Then, 100 µL of the oral fluid/buffer mixture was added to a 2 mL microcentrifuge tube; 20 µL of internal standard was also added, and the sample was vortexed for 10 seconds. Next, 460 µL of 90:10 0.1% formic acid in water:0.1% formic acid in methanol was added. Samples were vortexed again, and an aliquot was transferred to a 50 µL vial insert (cat.# 24513) in a 2.0 mL amber short-cap vial (cat.# 21142) and capped with a 9 mm short screw cap (cat.# 24497), then moved to the LC-MS/MS for analysis.
Supported Liquid Extraction
For the SLE samples, 1 mL of prepared calibrator or QC sample was added to 3 mL of Quantisal buffer to simulate real-world ratios. Then, 100 µL of the oral fluid/buffer mixture was added to a 2 mL microcentrifuge tube along with 100 µL of 5% ammonium hydroxide and 20 µL of the internal standard. The sample was then vortexed, and 200 µL was loaded into a 200 mg Resprep SLE cartridge (cat.# 28302). (Note that Resprep SLE 96-well plates [cat.# 28304] can be used for high-throughput analysis.) Light vacuum was applied for a few seconds to initiate loading the sample into the sorbent bed. The sample was then allowed to absorb into the sorbent over a period of 5 minutes. After 5 minutes, the sample was eluted with two 500 µL aliquots of 95:5 dichloromethane:isopropanol. The sample was evaporated to dryness and then reconstituted in 100 µL of 90:10 0.1% formic acid in water:0.1% formic acid in methanol. Samples were vortexed and transferred to a 50 µL vial insert (cat.# 24513) in a 2.0 mL amber short-cap vial (cat.# 21142) and capped with a 9 mm short screw cap (cat.# 24497), then moved to the LC-MS/MS for analysis.
Salt-Assisted Liquid-Liquid Extraction
For the SALLE samples, 1 mL of prepared calibrator or QC sample was added to 3 mL of Quantisal buffer to simulate real-world ratios. Then, 100 µL of the oral fluid/buffer mixture was added to a 2 mL microcentrifuge tube along with 20 µL of internal standard, and the sample was vortexed for 10 seconds. Next, 100 µL of a saturated NaCl solution was added to the tube, and the sample was vortexed again for 10 seconds followed by the addition of 280 µL of acetonitrile. The sample was vortexed for 10 seconds and then centrifuged at 3700 rpm for 10 minutes. Then, 200 µL of the organic layer was aliquoted to a test tube, and the sample was evaporated to dryness under nitrogen. Finally, the sample was reconstituted in 50 µL of 90:10 0.1% formic acid in water:0.1% formic acid in methanol. Samples were vortexed and transferred to a 50 µL vial insert (cat.# 24513) in a 2.0 mL amber short-cap vial (cat.# 21142) and capped with a 9 mm short screw cap (cat.# 24497), then moved to the LC-MS/MS for analysis.
Collection Kit Sample Volume Recovery
In order to assess real-world scenarios using sample collection devices, samples were tested using Quantisal kits. In these kits, the sponge was submerged in 1 mL of certified blank synthetic oral fluid that had been fortified at 150 ng/mL. Once the full 1 mL was absorbed, the sponge was submerged into the buffer solution and placed in the refrigerator overnight. The samples then underwent the same SALLE workflow described above.
Instrument Conditions
Oral fluid samples were analyzed for drugs of abuse on a Shimadzu Nexera X2 LC paired with a SCIEX 4500 MS/MS detector. The ions monitored for each compound are given in Table II. Instrument conditions are presented below.
Analytical column: Raptor Biphenyl 2.7 µm, 50 mm x 2.1 mm (cat.# 9309A52)
Guard column: Raptor Biphenyl EXP guard column cartridge 5 x 2.1 mm, 2.7 µm (cat # 9309A0252)
Mobile phase A: 0.1% Formic acid in water
Mobile phase B: 0.1% Formic acid in methanol
Gradient:
| Time (min) | %B |
|---|---|
| 0.00 | 15 |
| 1.00 | 20 |
| 2.00 | 20 |
| 4.00 | 50 |
| 6.00 | 60 |
| 8.00 | 100 |
| 9.00 | 100 |
| 9.01 | 15 |
| 10.00 | 15 |
Flow rate: 0.5 mL/min
Injection volume: 5 µL
Column temp.: 40 °C
Ion mode: Positive ESI
Table II: Target Drugs of Abuse and Ions for LC-MS/MS Analysis
| Analyte | tR | Precursor Ion | Product Ion 1 | Product Ion 2 |
|---|---|---|---|---|
| Morphine | 0.82 | 286.10 | 152.10 | 165.00 |
| Pregabalin | 0.88 | 160.18 | 55 | 97.1 |
| Oxymorphone | 0.92 | 302.14 | 227.20 | 198.20 |
| Cathinone | 0.94 | 149.90 | 132.90 | 106.20 |
| Amphetamine | 1.18 | 136.22 | 91.00 | 65.10 |
| Hydromorphone | 1.22 | 286.16 | 184.90 | 156.90 |
| Gabapentin | 1.22 | 172.20 | 154.00 | 137.10 |
| Methcathinone | 1.33 | 164.50 | 146.15 | 131.20 |
| MDA | 1.50 | 180.13 | 163.10 | 105.20 |
| Methamphetamine | 1.53 | 150.25 | 91.10 | 119.00 |
| Phentermine | 1.78 | 150.24 | 91.10 | 133.10 |
| Methylone | 1.80 | 207.90 | 159.90 | 189.90 |
| Lidocaine | 1.83 | 235.40 | 86.00 | 58.20 |
| Naloxone | 1.90 | 328.27 | 310.10 | 212.10 |
| Dihydrocodeine | 2.09 | 302.20 | 199.00 | 128.20 |
| MDMA | 2.15 | 194.17 | 163.00 | 105.1 |
| Codeine | 2.22 | 300.16 | 152.00 | 165.10 |
| 6-Acetylmorphine | 2.38 | 328.30 | 164.90 | 211.10 |
| Levamisole | 2.59 | 205.08 | 178.00 | 91.10 |
| Oxycodone | 2.69 | 316.19 | 298.00 | 169.00 |
| Naltrexone | 2.85 | 342.19 | 324.00 | 267.00 |
| MDEA | 2.92 | 208.13 | 163.00 | 105.20 |
| Hydrocodone | 2.98 | 300.14 | 199.00 | 128.00 |
| Norketamine | 3.30 | 224.08 | 125.00 | 89.10 |
| Eutylone | 3.39 | 236.10 | 188.05 | 218.15 |
| Norfentanyl | 3.55 | 233.13 | 84.10 | 55.00 |
| 4-Hydroxy nitazene | 3.70 | 369.10 | 100.20 | 72.20 |
| Pentylone | 3.73 | 236.10 | 188.05 | 218.15 |
| Dextrorphan | 3.78 | 258.15 | 157.00 | 201.20 |
| Xylazine | 3.85 | 221.90 | 90.10 | 71.90 |
| Ketamine | 3.90 | 238.07 | 125.10 | 89.10 |
| Benzoylecgonine | 4.00 | 290.13 | 168.10 | 77.10 |
| Meperidine | 4.03 | 248.14 | 220.10 | 174.10 |
| 7-Aminoclonazepam | 4.05 | 286.08 | 121.20 | 250.10 |
| Cocaine | 4.28 | 304.18 | 182.00 | 77.10 |
| 7-Hydroxymitragynine | 4.50 | 415.60 | 189.90 | 175.00 |
| Cocaethylene | 4.60 | 318.21 | 196.10 | 82.00 |
| LSD | 4.60 | 324.27 | 223.10 | 208.00 |
| Norbuprenorphine | 4.65 | 414.30 | 250.10 | 187.10 |
| Chlordiazepoxide | 4.74 | 300.10 | 282.00 | 227.00 |
| Acetyl fentanyl | 4.75 | 323.28 | 188.00 | 105.00 |
| Zolpidem | 4.80 | 308.21 | 235.20 | 218.90 |
| Fentanyl | 5.15 | 337.29 | 188.00 | 105.10 |
| Dextromethorphan | 5.17 | 272.19 | 215.10 | 170.90 |
| Isotonitazene | 5.18 | 411.00 | 100.20 | 72.15 |
| PCP | 5.20 | 244.15 | 86.10 | 159.10 |
| Buprenorphine | 5.20 | 468.30 | 396.20 | 414.20 |
| Midazolam | 5.22 | 326.19 | 291.10 | 248.90 |
| Propoxyphene | 5.33 | 340.27 | 265.90 | 58.10 |
| Tianeptine | 5.38 | 437.90 | 293.20 | 229.10 |
| Protonitazene | 5.49 | 411.00 | 100.30 | 72.15 |
| Sufentanil | 5.62 | 387.23 | 111.10 | 234.30 |
| EDDP | 5.62 | 278.15 | 234.30 | 249.20 |
| Mitragynine | 5.78 | 399.25 | 159.00 | 153.90 |
| iso-Butonitazene | 6.10 | 425.00 | 100.30 | 72.20 |
| Methadone | 6.25 | 310.20 | 264.90 | 105.10 |
| Lorazepam | 6.35 | 321.05 | 275.00 | 229.00 |
| Oxazepam | 6.40 | 287.07 | 268.80 | 241.20 |
| Clonazepam | 6.50 | 316.09 | 270.00 | 214.10 |
| Nordiazepam | 6.80 | 271.00 | 139.90 | 164.90 |
| Clonazolam | 6.83 | 354.10 | 308.10 | 279.80 |
| Alprazolam | 7.28 | 309.11 | 280.90 | 204.90 |
| Temazepam | 7.30 | 301.11 | 255.10 | 282.90 |
| Bromazolam | 7.40 | 353.10 | 325.20 | 218.00 |
| Etizolam | 7.52 | 343.10 | 314.00 | 258.90 |
| Diazepam | 7.59 | 285.07 | 153.90 | 192.90 |
| Cannabidiol | 7.85 | 315.20 | 193.00 | 123.20 |
| Delta-9-THC | 8.10 | 315.50 | 193.10 | 259.10 |
Results and Discussion
Optimization of Elution Solvent for SLE
Finding the right elution solvent is a primary concern when using SLE as a sample preparation technique. Because this method included a large list of analytes that varied in polarity, an elution solvent optimization study was performed. Samples were extracted using the SLE workflow, and seven different elution solvents were tested. The elution solvents tested were methanol; acetonitrile; dichloromethane; hexane; 50:50 dichloromethane:ethyl acetate; 95:5 dichloromethane:isopropanol; and 95:5 dichloromethane:methanol. Samples were tested at the low and high QC levels and peak shape, peak area, and peak height were compared for all analytes. Based on the results, 95:5 dichloromethane:isopropanol showed the best overall performance and was chosen as the elution solvent.
Sample Preparation Comparison
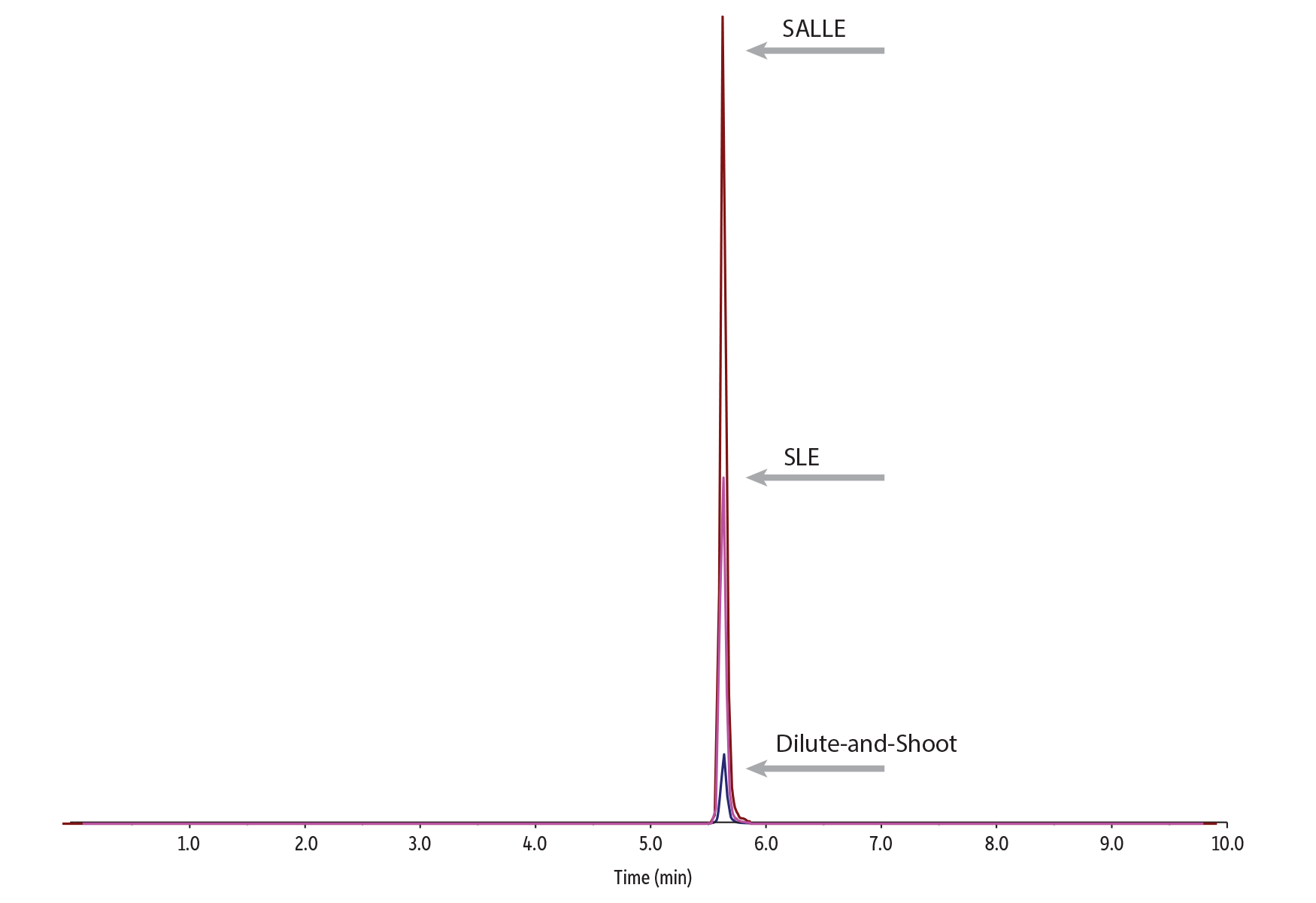
Both sample preparation techniques (SLE and SALLE) showed significant improvements in analyte response compared to the dilute-and-shoot technique. SLE worked well for cleaning up samples that shared similar analyte properties, but SALLE produced better results across the full list of compounds, which included drugs with a wider range of characteristics. The SLE sample preparation used in this workflow showed improved response for most opiates; however, it did result in unacceptably low responses for pregabalin, gabapentin, THC, and CBD. Therefore, these analytes were not included in the accuracy and precision experiments for the SLE workflow. In Figure 1, peak area responses for all three sample preparations are compared at 10 ng/mL.
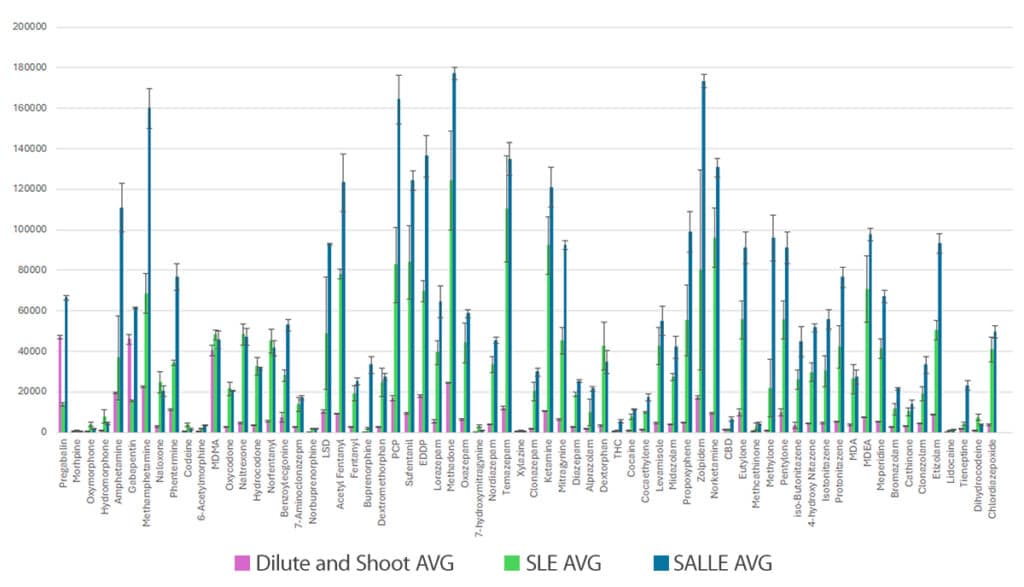
Error bars represent standard deviations.
To further demonstrate the performance benefits of SALLE and SLE compared to a dilute-and-shoot approach, peak responses for mitragynine and norfentanyl were compared. Mitragynine showed the highest response when using SALLE (Figure 2) while norfentanyl had the higher response when using SLE (Figure 3). While both cleanup techniques provided dramatic improvements over dilute-and-shoot, these examples also highlight that the most effective sample preparation approach can vary by analyte, so the analyte list and LOQ requirements should be carefully considered when choosing a technique.
Collection Kit Sample Volume Recovery
When using oral fluid collection kits, it is very important to remove the matrix and buffer from the sponge in the collection device. There are two main ways to empty the sponge: manual compression and centrifugation. Figure 4 shows that centrifugation resulted in near maximum recovery of the expected 4 mL volume, whereas compression recovered approximately 3 mL of the expected amount.

It is clear that the centrifugation technique is more effective at extracting all of the oral fluid and buffer from the sponge. However, some precautions must be taken because the sponge can become fragile after sitting in the buffer and can easily detach from the applicator if centrifuged at high speeds. In order to avoid this, it is important to fold the sponge against the side of the tube prior to centrifugation.
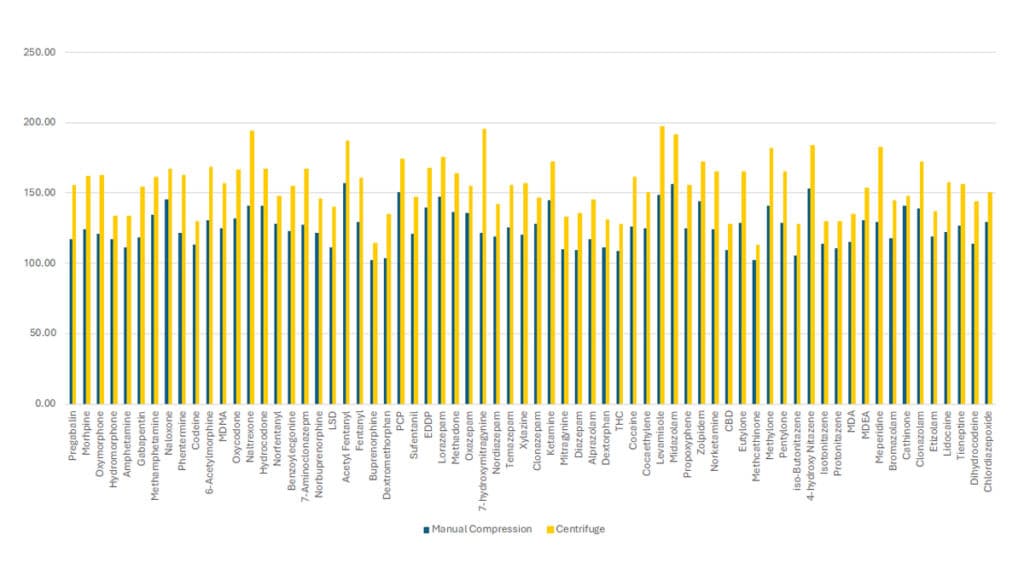
To show the effect that sample volume recovery has on analyte recovery, medium QC level (150 ng/mL) samples were processed using either the manual compression technique or the centrifuge technique. All 68 analytes in the method showed an increase in recovery when using the centrifugation technique (Figure 5).
Chromatographic Performance
As shown in Figure 6, pairing the SALLE sample preparation technique with the chromatographic conditions used here, all 68 drugs of abuse, including novel psychoactive substances, were successfully analyzed in oral fluid in a fast, 10-minute cycle time on a Raptor Biphenyl 50 x 2.1 mm, 2.7 µm column by LC-MS/MS. In addition, the method provided adequate chromatographic separation of all isobars, allowing accurate quantification.
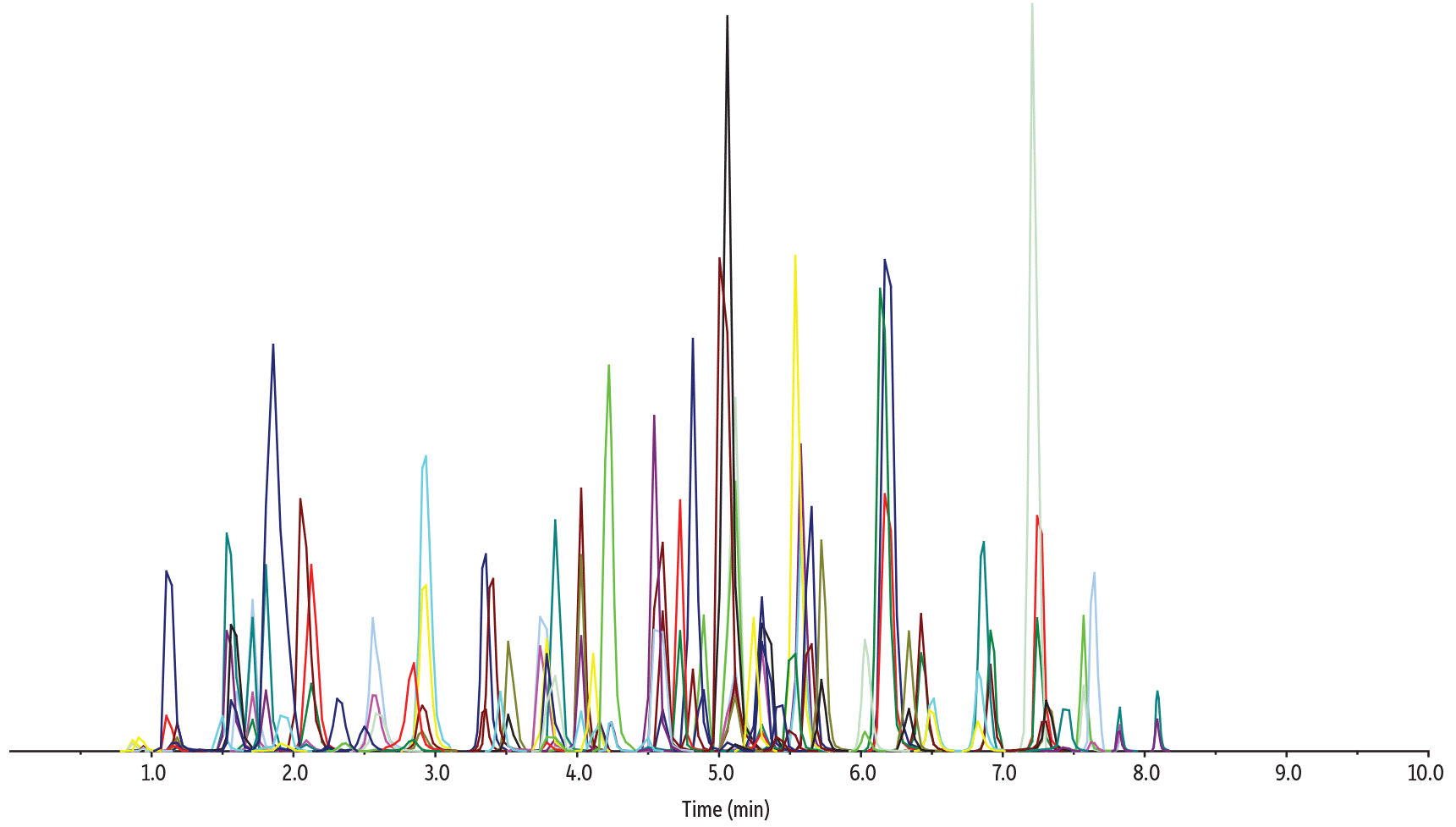
LC_CF0830
Peaks
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion 1 | Product Ion 2 | |
|---|---|---|---|---|---|---|
| 1. | Morphine | 0.82 | 100 | 286.1 | 152.1 | 165.0 |
| 2. | Pregabalin | 0.88 | 1000 | 160.1 | 55 | 97.1 |
| 3. | Oxymorphone | 0.92 | 100 | 302.1 | 227.2 | 198.2 |
| 4. | Cathinone | 0.94 | 100 | 149.9 | 132.9 | 106.2 |
| 5. | Amphetamine | 1.22 | 100 | 136.2 | 91.0 | 65.1 |
| 6. | Hydromorphone | 1.22 | 100 | 286.1 | 184.9 | 156.9 |
| 7. | Gabapentin | 1.22 | 1000 | 172.2 | 154.0 | 137.1 |
| 8. | Methcathinone | 1.33 | 100 | 164.5 | 146.1 | 131.2 |
| 9. | MDA | 1.50 | 100 | 180.1 | 105.2 | 163.1 |
| 10. | Methamphetamine | 1.53 | 100 | 150.2 | 119.0 | 91.1 |
| 11. | Phentermine | 1.78 | 100 | 150.2 | 133.1 | 91.1 |
| 12. | Methylone | 1.80 | 100 | 207.9 | 159.9 | 189.9 |
| 13. | Lidocaine | 1.83 | 100 | 235.4 | 86.0 | 58.2 |
| 14. | Naloxone | 1.90 | 100 | 328.2 | 310.1 | 212.1 |
| 15. | Dihydrocodeine | 2.09 | 100 | 302.2 | 199.0 | 128.2 |
| 16. | MDMA | 2.15 | 100 | 194.1 | 163.0 | 105.1 |
| 17. | Codeine | 2.22 | 100 | 300.1 | 152.0 | 165.1 |
| 18. | 6-Acetylmorphine | 2.38 | 100 | 328.3 | 164.9 | 211.1 |
| 19. | Levamisole | 2.59 | 100 | 205.1 | 178.0 | 91.1 |
| 20. | Oxycodone | 2.69 | 100 | 316.1 | 298.0 | 169.0 |
| 21. | Naltrexone | 2.85 | 100 | 342.1 | 324.0 | 267.0 |
| 22. | MDEA | 2.92 | 100 | 208.1 | 163.0 | 105.2 |
| 23. | Hydrocodone | 2.98 | 100 | 300.1 | 199.0 | 128.0 |
| 24. | Norketamine | 3.30 | 100 | 224.1 | 125.0 | 89.1 |
| 25. | Eutylone | 3.39 | 100 | 236.1 | 188.1 | 218.1 |
| 26. | Norfentanyl | 3.55 | 100 | 233.1 | 84.1 | 55.0 |
| 27. | 4′-Hydroxy nitazene | 3.70 | 100 | 369.1 | 100.2 | 72.2 |
| 28. | Pentylone | 3.78 | 100 | 236.1 | 188.0 | 218.1 |
| 29. | Dextrorphan | 3.78 | 100 | 258.1 | 157.0 | 201.2 |
| 30. | Xylazine | 3.85 | 100 | 221.9 | 90.1 | 71.9 |
| 31. | Ketamine | 3.90 | 100 | 238.1 | 125.1 | 89.1 |
| 32. | Benzoylecgonine | 4.00 | 100 | 290.1 | 168.1 | 77.1 |
| 33. | Meperidine | 4.03 | 100 | 248.1 | 220.1 | 174.1 |
| 34. | 7-Aminoclonazepam | 4.05 | 100 | 286.1 | 121.2 | 250.1 |
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion 1 | Product Ion 2 | |
|---|---|---|---|---|---|---|
| 35. | Cocaine | 4.28 | 100 | 304.1 | 182.0 | 77.1 |
| 36. | 7-Hydroxymitragynine | 4.50 | 100 | 415.6 | 189.9 | 175.0 |
| 37. | LSD | 4.60 | 100 | 324.2 | 223.1 | 208.0 |
| 38. | Cocaethylene | 4.60 | 100 | 318.2 | 196.1 | 82.0 |
| 39. | Norbuprenorphine | 4.65 | 100 | 414.3 | 187.1 | 210.9 |
| 40. | Chlordiazepoxide | 4.74 | 100 | 300.1 | 282.0 | 227.0 |
| 41. | Acetyl fentanyl | 4.75 | 100 | 323.2 | 188.0 | 105.0 |
| 42. | Zolpidem | 4.80 | 100 | 308.2 | 235.2 | 218.9 |
| 43. | Fentanyl | 5.15 | 100 | 337.2 | 188.0 | 105.1 |
| 44. | Dextromethorphan | 5.17 | 100 | 272.1 | 215.1 | 170.9 |
| 45. | Isotonitazene | 5.18 | 100 | 411.0 | 100.2 | 72.1 |
| 46. | Buprenorphine | 5.20 | 100 | 468.3 | 396.2 | 414.2 |
| 47. | PCP | 5.20 | 100 | 244.1 | 86.1 | 159.1 |
| 48. | Midazolam | 5.22 | 100 | 326.1 | 291.1 | 248.9 |
| 49. | Propoxyphene | 5.33 | 100 | 340.2 | 265.9 | 58.1 |
| 50. | Tianeptine | 5.38 | 100 | 437.9 | 293.2 | 229.1 |
| 51. | Protonitazene | 5.49 | 100 | 411.0 | 100.3 | 72.1 |
| 52. | Sufentanil | 5.62 | 100 | 387.2 | 238.1 | 111.1 |
| 53. | EDDP | 5.62 | 100 | 278.1 | 234.3 | 249.2 |
| 54. | Mitragynine | 5.78 | 100 | 399.2 | 174.2 | 159.0 |
| 55. | iso-Butonitazene | 6.10 | 100 | 425.0 | 100.3 | 72.2 |
| 56. | Methadone | 6.25 | 100 | 310.2 | 264.9 | 105.1 |
| 57. | Lorazepam | 6.35 | 100 | 321.1 | 275.0 | 229.0 |
| 58. | Oxazepam | 6.40 | 100 | 287.0 | 268.8 | 241.2 |
| 59. | Clonazepam | 6.50 | 100 | 316.1 | 270.0 | 214.1 |
| 60. | Nordiazepam | 6.80 | 100 | 271.0 | 139.9 | 164.9 |
| 61. | Clonazolam | 6.83 | 100 | 354.1 | 308.1 | 279.8 |
| 62. | Alprazolam | 7.28 | 100 | 309.1 | 280.9 | 204.9 |
| 63. | Temazepam | 7.30 | 100 | 301.1 | 255.1 | 282.9 |
| 64. | Bromazolam | 7.40 | 100 | 353.1 | 325.2 | 218.0 |
| 65. | Etizolam | 7.52 | 100 | 343.1 | 314.0 | 258.9 |
| 66. | Diazepam | 7.59 | 100 | 285.07 | 153.9 | 192.9 |
| 67. | Cannabidiol | 7.85 | 100 | 315.2 | 193.0 | 123.2 |
| 68. | d9-THC | 8.10 | 100 | 315.5 | 193.1 | 259.1 |
Conditions
| Column | Raptor Biphenyl (cat.# 9309A52) | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 50 mm x 2.1 mm ID | ||||||||||||||||||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||||||||||||||||||
| Guard Column: | Raptor Biphenyl 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9309A0252) | ||||||||||||||||||||||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||||||||||||||||||
| Diluent: | 90% Water, 0.1 % formic acid:10% methanol, 0.1% formic acid | ||||||||||||||||||||||||||||||||||||||||
| Inj. Vol.: | 5 µL | ||||||||||||||||||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||||||||||||||||||
| A: | Water, 0.1 % formic acid | ||||||||||||||||||||||||||||||||||||||||
| B: | Methanol, 0.1% formic acid | ||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||
| Max Pressure: | 310 bar |
| Detector | Sciex 4500 MS/MS |
|---|---|
| Interface: | ESI+ |
| Instrument | Shimadzu Nexera X2 |
| Sample Preparation | One milliliter of synthetic oral fluid fortified with 100 µL of calibrator/QC standard was added to 3 mL of Quantisal buffer to simulate real-world ratios. One hundred microliters of the oral fluid/buffer mixture was added to a 2 mL microcentrifuge tube along with 20 µL of internal standard. The sample was vortexed for 10 seconds. One hundred microliters of a saturated NaCl solution was added to the tube, and the sample was vortexed again for 10 seconds. Two-hundred and eighty microliters of acetonitrile was added to the sample. The sample was vortexed for 10 seconds and then centrifuged at 3700 rpm for 10 minutes. Two hundred microliters of the organic layer was aliquoted to a test tube, and the sample was evaporated to dryness under nitrogen. The sample was then reconstituted in 50 µL of 90:10 0.1% formic acid in water:0.1% formic acid in methanol. Samples were vortexed and added to a 50 µL vial insert (cat.# 24513) in a 2.0 mL, amber, short-cap vial (cat.# 21142) and capped with a 9 mm short cap (cat.# 24497), then moved to the LC-MS/MS for analysis. |
Linearity
Linearity was demonstrated using a 1/x weighted linear regression, and all analytes showed acceptable R2 values of 0.991 or greater for both sample preparation techniques. The analytical ranges for the target drugs of abuse are presented in Table III.
Table III: Analytical Ranges in Oral Fluid for All Analytes
| Analyte | Linear Range (ng/mL) | Analyte | Linear Range (ng/mL) |
|---|---|---|---|
| Morphine | 2-1000 | Cocaine | 2-1000 |
| Pregabalin | 50-10,000 | 7-hydroxymitragynine | 10-1000 |
| Oxymorphone | 2-1000 | LSD | 2-1000 |
| Cathinone | 2-1000 | Cocaethylene | 2-1000 |
| Amphetamine | 2-1000 | Norbuprenorphine | 2-1000 |
| Hydromorphone | 2-1000 | Chlordiazepoxide | 2-1000 |
| Gabapentin | 50-10,000 | Acetyl Fentanyl | 2-1000 |
| Methcathinone | 5-1000 | Zolpidem | 2-1000 |
| MDA | 2-1000 | Fentanyl | 2-1000 |
| Methamphetamine | 2-1000 | Dextromethorphan | 2-1000 |
| Phentermine | 2-1000 | Isotonitazene | 2-1000 |
| Methylone | 2-1000 | Buprenorphine | 2-1000 |
| Lidocaine | 2-1000 | PCP | 2-1000 |
| Naloxone | 2-1000 | Midazolam | 2-1000 |
| Dihydrocodeine | 5-1000 | Propoxyphene | 2-1000 |
| MDMA | 2-1000 | Tianeptine | 2-1000 |
| Codeine | 2-1000 | Protonitazene | 2-1000 |
| 6-Acetylmorphine | 2-1000 | Sufentanil | 2-1000 |
| Levamisole | 2-1000 | EDDP | 2-1000 |
| Oxycodone | 2-1000 | Mitragynine | 2-1000 |
| Naltrexone | 2-1000 | Iso-Butonitazene | 5-1000 |
| MDEA | 2-1000 | Methadone | 2-1000 |
| Hydrocodone | 2-1000 | Lorazepam | 2-1000 |
| Norketamine | 2-1000 | Oxazepam | 2-1000 |
| Eutylone | 2-1000 | Clonazepam | 2-1000 |
| Norfentanyl | 2-1000 | Nordiazepam | 5-1000 |
| 4-Hydroxy nitazene | 2-1000 | Clonazolam | 2-1000 |
| Pentylone | 2-1000 | Alprazolam | 2-1000 |
| Dextrorphan | 5-1000 | Temazepam | 2-1000 |
| Xylazine | 5-1000 | Bromazolam | 2-1000 |
| Ketamine | 5-1000 | Etizolam | 2-1000 |
| Benzoylecgonine | 2-1000 | Diazepam | 2-1000 |
| Meperidine | 2-1000 | Delta-9-THC | 5-1000 |
| 7-Aminoclonazepam | 5-1000 | Cannabidiol | 5-1000 |
Accuracy and Precision
Precision and accuracy analyses were performed over the course of multiple days using both sample preparation methods. Method accuracy was successfully demonstrated for both techniques with QC LOQ, QC low, QC medium, and QC high values falling within ±15% of the expected concentrations. Intraday and interday precision was also acceptable with %RSDs of ≤9.12% for SLE and ≤7.45% for SALLE.
Column Robustness
Column robustness was tested by running more than 250 consecutive matrix injections of both SLE and SALLE prepared samples. The Raptor Biphenyl column produced highly consistent results, and the retention times of the first and last injections were ≤6.21% different for all 68 drugs of abuse.
Conclusion
The method developed here allows for a quick and efficient sample preparation and analysis of 68 drugs of abuse and novel psychoactive substances in oral fluid by LC-MS/MS. Separation of isobars was achieved in this robust 10-minute method, allowing high-throughput quantitative analysis. This method demonstrated successful precision, accuracy, and linearity for all analytes using SALLE and for most analytes using SLE. It also demonstrated that SALLE worked better for the broader list of analytes while SLE worked well for specific groups of analytes. This allows laboratories to make decisions on sample preparation techniques depending on their analyte list and workflow requirements. Both sample preparation methods showed increases in analyte recovery compared to dilute-and-shoot, as well as effective cleanup of buffer additives, allowing for longer column lifetime and no adverse method effects downstream. The SALLE and SLE procedures used here were both simple and quick to perform, which is advantageous compared to lengthy, more complex SPE procedures that are sometimes used for analyzing drugs of abuse in oral fluids.
References
- E.J. Cone, M.A. Huestis, Marilyn, Interpretation of oral fluid tests for drugs of abuse, Ann NY Acad Sci, 1098 (2007) 51-103. https://doi.org/10.1196/annals.1384.037
- S. Uljon, Chapter One-Advances in fentanyl testing, Advances in Clinical Chemistry, 116 (2023)1-30. https://doi.org/10.1016/bs.acc.2023.05.004
This method has been developed for research use only; it is not suitable for use in diagnostic procedures without further evaluation.