Abstract
PFAS analysis in nondrinking water matrices presents unique challenges to sample preparation. Trace-level analysis of PFAS in surface water and wastewater requires both low background contamination and the ability to handle challenging matrices. Resprep PFAS cartridges allow fast, reliable SPE sample preparation of non-potable waters, such as those covered in EPA Method 1633 for PFAS analysis. The inclusion of both WAX and carbon layers in a single cartridge allows for simultaneous extraction and cleanup. In addition, an optional filter aid prevents the cartridges from clogging when preparing samples that contain high amounts of suspended solids.
Introduction
While methods for PFAS analysis in drinking water have been widely used for some time, the absence of methods for non-potable waters using solid-phase extraction (SPE) has led many labs to modify drinking water methods when analyzing surface water and wastewater. To address this, EPA Method 1633 [1], which was finalized in January of 2024, provides a sample preparation approach that includes an SPE extraction using a weak anion exchange (WAX) cartridge manually packed with glass wool and a dispersive carbon cleanup. The manual packing of glass wool and separate carbon cleanup step add extra time, complexity, and risk of introducing contaminants to the sample preparation process, and manually packed wool can increase variation among technicians. Labs interested in streamlining their sample preparation workflows can utilize Resprep PFAS cartridges, which are dual-bed SPE cartridges that contain both WAX and carbon sorbent beds that allow the SPE extraction and carbon cleanup to be accomplished in a single cartridge. An optional filter aid eliminates the need for manual addition of glass wool to handle samples containing solids, and it provides much better performance in preventing clogs.
Experimental
Sample Preparation
Samples for precision, accuracy, and method detection limit (MDL) studies were prepared in polypropylene bottles using 500 mL deionized water spiked with 25 µL of extracted internal standards (EIS, Wellington Laboratories p/n MPFAC-HIF-ES) as per EPA Method 1633, Section 11.2.4. Four samples for precision and recovery analysis were spiked with 200 µL of native PFAS standards (Wellington Laboratories p/n EPA-1633STK), giving pre-extraction concentrations of 100-2500 ng/L. Seven MDL samples were spiked with 20 µL of a 20:1 dilution of the native standard, giving pre-extraction concentrations of 0.5-12.5 ng/L. Seven blank replicates were prepared for the MDL study as well. The MDL samples were prepared and analyzed over three days.
Resprep PFAS SPE cartridges (6 mL), which contained 150 mg of 30 µm WAX and 50 mg of CarboPrep Plus carbon (cat.# 28930), were placed on a Thermo AutoTrace PFAS system. After preparation of the samples and setup of the SPE system, the samples were extracted following the instructions in EPA Method 1633, Section 11.4, which is summarized in Figure 1. After extraction, the extracts were spiked with 25 µL of non-extracted internal standards (NIS, Wellington Laboratories p/n MPFAC-HIF-IS).
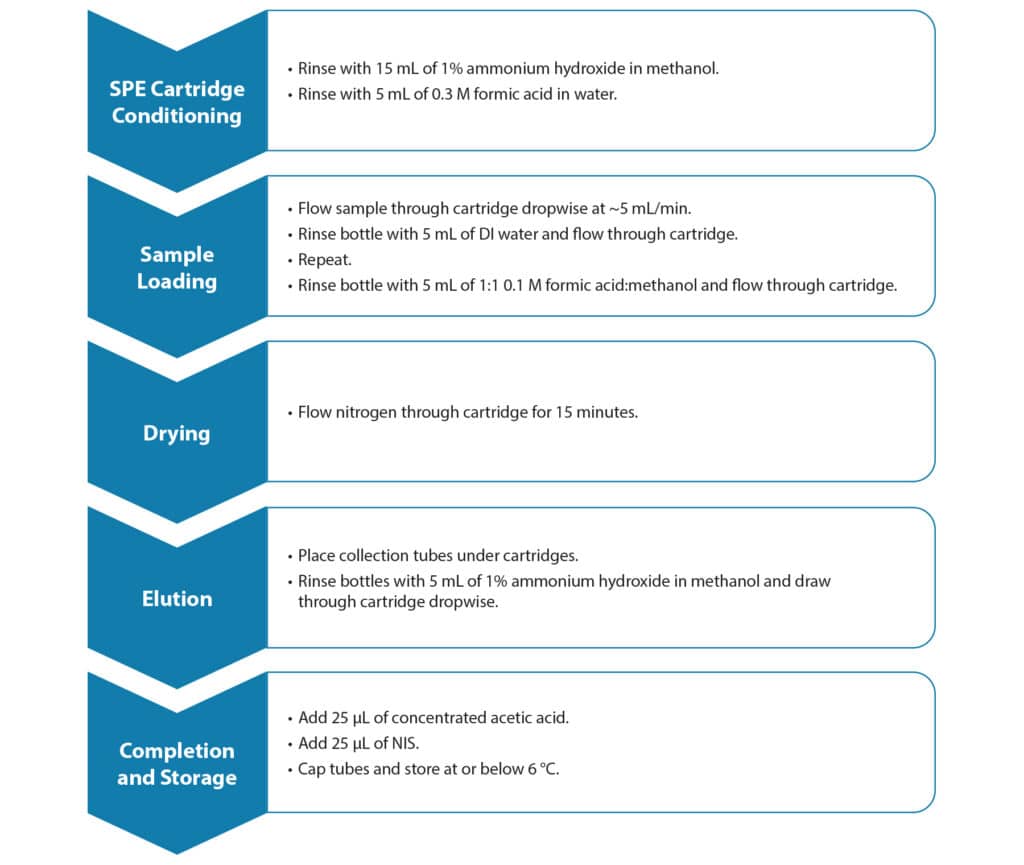
Samples to test filter aid efficiency were prepared using ASTM substitute wastewater [2] diluted by 2.5x to be ~100 mg/L suspended solids. Resprep PFAS SPE cartridges (cat.# 28931), which contained 2000 mg of filter aid, 150 mg of 30 µm WAX, and 50 mg of CarboPrep Plus carbon, were placed on a Resprep QR-12 vacuum manifold (cat.# 28298-VM) that was fitted with quick-replace liners (cat.# 28310-VM). Resprep sample delivery system lines (cat.# 26250) were used to transfer the samples to the SPE cartridges. Five hundred milliliters of the substitute wastewater was loaded onto the cartridge. The volume of sample that passed through without clogging was compared to cartridges containing only WAX (cat.# 28292) and WAX cartridges manually packed with glass wool (cat.# 24324) to half the height of the SPE cartridge as described in Method 1633, Section 12.1.1.
Analytical System
After extraction, the samples were analyzed by LC-MS/MS under the conditions shown below. The use of a PFAS delay column is important to prevent any PFAS contamination upstream of the injector from coeluting with the samples. Thorough blank checking of the analytical system was performed and showed no detectable PFAS contamination.
Instrument Conditions for EPA Method 1633 PFAS Analysis
System: Waters ACQUITY Premier LC/Xevo TQ Absolute Triple Quadrupole MS
Columns:
• PFAS delay column (cat.# 27854)
• Analytical column: Force C18, 1.8 µm x 50 mm x 2.1 mm (cat.# 9634252)
Injection volume: 3 µL
Mobile phase A: Water, 5 mM ammonium acetate
Mobile phase B: Methanol
Flow rate: 0.4 mL/min
Temperature: 40 °C
Gradient:
| Time (min) | %B |
| 0 | 20 |
| 6 | 95 |
| 6.6 | 95 |
| 6.61 | 20 |
| 7.5 | 20 |
Ion source: Electrospray
Ion mode: ESI-
Mode: MRM
The analytical system was calibrated using the concentrations shown in Table 4 of Method 1633 for PFAS analysis. Curve fits were chosen that minimized % relative standard error (%RSE).
Method Detection Limits (MDL)
The method detection limit was calculated from the analysis of seven blank replicates and seven low-level spikes, as outlined in EPA’s Definition and Procedure for the Determination of the Method Detection Limit, Revision 2 [3]. The standard deviations of the spike and blank results were multiplied by the Student’s t-value of 3.143 to determine the MDL, and the higher of the results between the spikes and blanks was selected as the MDL.
Accuracy and Precision
Accuracy and precision were determined by analyzing four replicate spikes. The accuracy of the spikes was calculated and compared to the recovery limits in Table 5 of EPA Method 1633. The relative standard deviations of the spike results were also determined and compared to the limits in Table 5. The recovery of the isotope dilution standards was calculated from the spike replicates and compared to the recovery limits from Table 6 of EPA Method 1633 for PFAS analysis.
Results and Discussion
Method Performance Verification Experiment
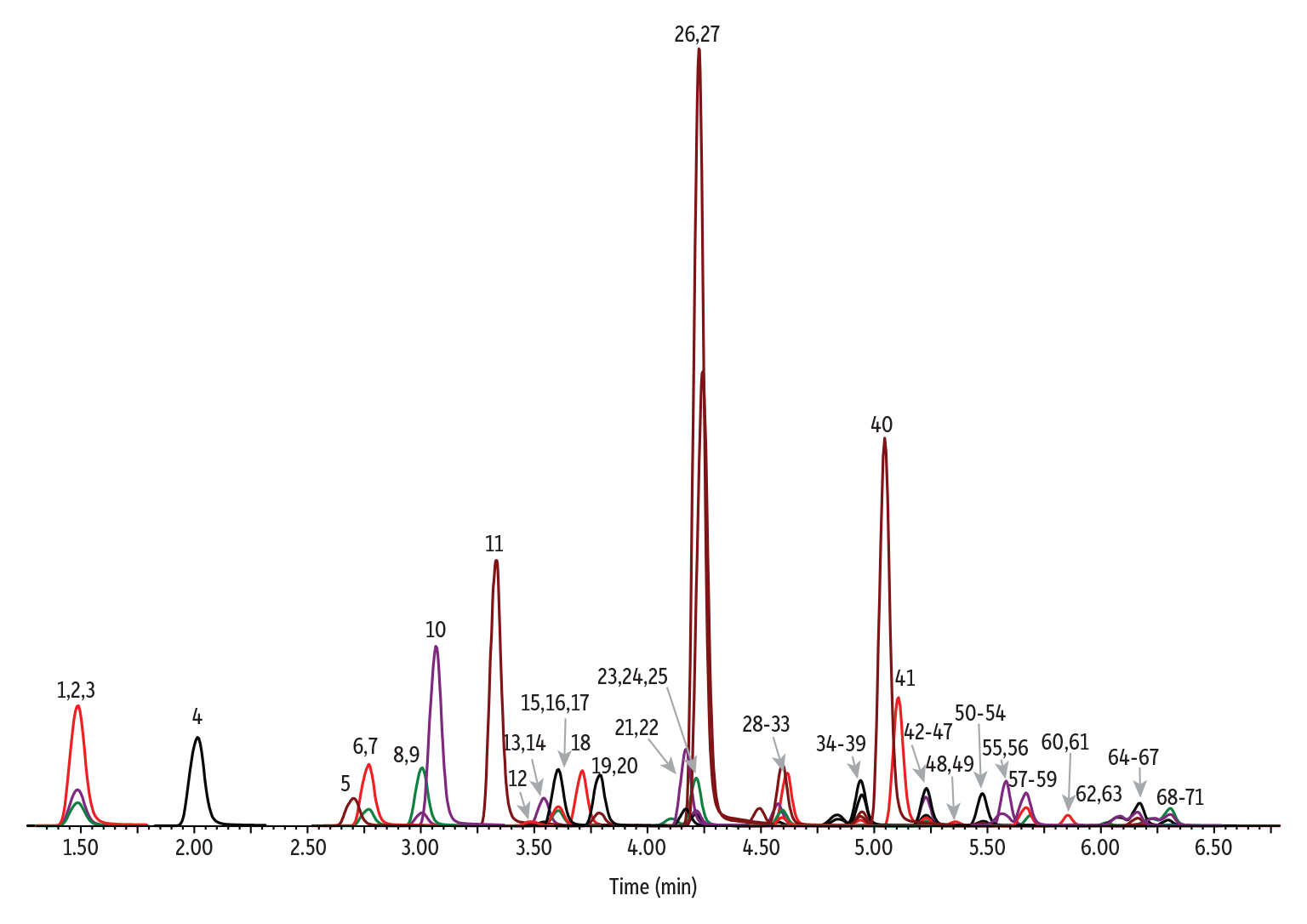
Good chromatographic results were obtained for all compounds, as shown in Figure 2. The MDL, blank levels, accuracy, and precision results for native PFAS analytes are shown in Table I. The calculated MDLs were below the reporting limits shown in Table 9 in EPA Method 1633, and the accuracy of the spikes ranged from 77 to 125% of the spiked value, meeting the requirements in Table 5 of Method 1633. The spikes showed good precision as well, with the results being ≤25% RSD. Similarly, the recoveries for the isotope dilution standards were also within the limits listed in Table 6 of the method. The results are shown in Table II.
LC_EV0599
Peaks
| Peaks | tR (min) | Conc. (ng/L) | Precursor 1 | Product 1 | Product 2 | Precursor 2 | Product 1 | |
|---|---|---|---|---|---|---|---|---|
| 1. | Perfluoro-n-[1,2,3,4- 13C3]butanoic acid (13C3-PFBA) | 1.485 | 5 | 216 | 172 | – | – | – |
| 2. | Perfluoro-n-[1,2,3,4- 13C4]butanoic acid (13C4-PFBA) | 1.487 | 10 | 217 | 172 | – | – | – |
| 3. | Perfluorobutanoic acid (PFBA) | 1.489 | 10 | 213 | 169 | – | – | – |
| 4. | Perfluoro-3-methoxypropanoic acid (PFMPA) | 2.007 | 5 | 229 | 85 | – | – | – |
| 5. | 3-Perfluoropropyl propanoic acid (3:3FTCA) | 2.7 | 12.5 | 241 | 177 | 117 | – | – |
| 6. | Perfluoro-n-[1,2,3,4,5- 13C5]pentanoic acid (13C5-PFPeA) | 2.765 | 5 | 268 | 223 | – | – | – |
| 7. | Perfluoropentanoic acid (PFPeA) | 2.768 | 5 | 263 | 219 | 69 | – | – |
| 8. | Perfluorobutane sulfonate (PFBS) | 3.006 | 2.5 | 299 | 80 | 99 | – | – |
| 9. | Sodium perfluoro-1-[2,3,4- 13C3]butanesulfonate (13C3-PFBS) | 3.007 | 2.5 | 302 | 80 | 99 | – | – |
| 10. | Perfluoro-4-methoxybutanoic acid (PFMBA) | 3.071 | 5 | 279 | 85 | – | – | – |
| 11. | Perfluoro(2-ethoxyethane)sulfonic acid (PFEESA) | 3.333 | 5 | 315 | 135 | 83 | – | – |
| 12. | Nonafluoro-3,6-dioxaheptanoic acid (NFDHA) | 3.493 | 5 | 295 | 201 | 85 | – | – |
| 13. | 1H,1H,2H,2H-perfluorohexane sulfonate (4:2 FTS) | 3.545 | 10 | 327 | 307 | 81 | – | – |
| 14. | Sodium 1H,1H,2H,2H-perfluoro-1-[1,2- 13C2]hexane sulfonate (13C2-4:2FTS) | 3.55 | 5 | 329 | 81 | 309 | – | – |
| 15. | Perfluorohexanoic acid (PFHxA) | 3.608 | 2.5 | 313 | 269 | 119 | – | – |
| 16. | Perfluoro-n-[1,2,3,4,6- 13C2]hexanoic acid (13C2-PFHxA) | 3.609 | 2.5 | 315 | 270 | 119 | – | – |
| 17. | Perfluoro-n-[1,2,3,4,6- 13C5]hexanoic acid (13C5-PFHxA) | 3.61 | 2.5 | 318 | 273 | 120 | – | – |
| 18. | Perfluoropentane sulfonate (PFPeS) | 3.718 | 2.5 | 349 | 80 | 99 | – | – |
| 19. | 2,3,3,3-Tetrafluoro-2-(1,1,2,2,3,3,3-heptafluoropropoxy13C3-propanoic acid (13C3-HFPO-DA) | 3.79 | 10 | 287 | 169 | 185 | – | – |
| 20. | Hexafluoropropylene oxide (HFPO-DA) | 3.795 | 10 | 285 | 169 | 185 | – | – |
| 21. | Perfluoroheptanoic acid (PFHpA) | 4.174 | 2.5 | 363 | 319 | 169 | – | – |
| 22. | Perfluoro-n-[1,2,3,4- 13C4]heptanoic acid (13C4-PFHpA) | 4.174 | 2.5 | 367 | 322 | – | – | – |
| 23. | Perfluoro-1-hexane[18O2]sulfonic acid (18O2-PFHxS) | 4.218 | 2.5 | 403 | 84 | – | – | – |
| 24. | Sodium perfluoro-1-[1,2,3- 13C3]hexanesulfonate (13C3-PFHxS) | 4.218 | 2.5 | 402 | 80 | 99 | – | – |
| 25. | Perfluorohexane sulfonate (PFHxS) | 4.22 | 2.5 | 399 | 80 | 99 | – | – |
| 26. | <a class="cmpd_link" title="View compound information for 4,8-Dioxa-3H-perfluorononanoic acid (ADONA)” title=”View compound information for 4,8-Dioxa-3H-perfluorononanoic acid (ADONA)” href=”https://ez.restek.com/compound/view/en/919005-14-4/4,8-Dioxa-3H-perfluorononanoic acid”>4,8-Dioxa-3H-perfluorononanoic acid (ADONA) | 4.232 | 10 | 377 | 251 | 85 | – | – |
| 27. | 2H,2H,3H,3H-Perfluorooctanoic acid (5:3FTCA) | 4.247 | 62.5 | 341 | 237 | 217 | – | – |
| 28. | 1H,1H,2H,2H-perfluorooctane sulfonate (6:2 FTS) | 4.58 | 10 | 427 | 407 | 81 | – | – |
| 29. | Sodium 1H,1H,2H,2H-perfluoro-1-[1,2-13C2]-octane sulfonate (13C2-6:2FTS) | 4.581 | 5 | 429 | 81 | 409 | – | – |
| 30. | Perfluoro-n-[ 13C8]octanoic acid (13C8-PFOA) | 4.598 | 2.5 | 421 | 376 | – | – | – |
| 31. | Perfluorooctanoic acid (PFOA) | 4.601 | 2.5 | 413 | 369 | 169 | – | – |
| 32. | Perfluoro-n-[ 13C4]octanoic acid (13C4-PFOA) | 4.601 | 2.5 | 417 | 172 | – | – | – |
| 33. | Perfluoroheptane sulfonate (PFHpS) | 4.621 | 2.5 | 449 | 80 | 99 | – | – |
| 34. | Perfluorononanoic acid (PFNA) | 4.944 | 2.5 | 463 | 419 | 219 | – | – |
| 35. | Perfluoro-n-[ 13C5]nonanoic acid (13C5-PFNA) | 4.944 | 1.25 | 468 | 423 | – | – | – |
| 36. | Perfluoro-n-[ 13C9]nonanoic acid (13C9-PFNA) | 4.944 | 1.25 | 472 | 427 | – | – | – |
| 37. | Perfluorooctane sulfonate (PFOS) | 4.949 | 2.5 | 499 | 80 | 99 | – | – |
| 38. | Sodium perfluoro-[ 13C4]octanesulfonate (13C4-PFOS) | 4.95 | 2.5 | 503 | 80 | 99 | – | – |
| 39. | Sodium perfluoro-[ 13C8]octanesulfonate (13C8-PFOS) | 4.95 | 2.5 | 507 | 80 | 99 | – | – |
| 40. | 3-Perfluoroheptyl propanoic acid (7:3FTCA) | 5.046 | 62.5 | 441 | 317 | 337 | – | – |
| 41. | 9-Chlorohexadecafluoro-3-oxanonane-1-sulfonic acid (9Cl-PF3ONS) | 5.114 | 10 | 531 | 351 | – | 533 | 353 |
| 42. | Perfluorodecanoic acid (PFDA) | 5.237 | 2.5 | 513 | 469 | 219 | – | – |
| 43. | Perfluoro-n-[1,2,3,4,5,6- 13C2]decanoic acid (13C2-PFDA) | 5.237 | 1.25 | 515 | 470 | – | – | – |
| 44. | Perfluoro-n-[1,2,3,4,5,6- 13C6]decanoic acid (13C6-PFDA) | 5.237 | 1.25 | 519 | 474 | – | – | – |
| 45. | 1H,1H,2H,2H-perfluorodecane sulfonate (8:2 FTS) | 5.238 | 10 | 527 | 507 | 81 | – | – |
| 46. | Sodium 1H,1H,2H,2H-perfluoro-1-[1,2- 13C2]-decane sulfonate (13C2-8:2FTS) | 5.238 | 5 | 529 | 81 | 509 | – | – |
| 47. | Perfluorononanesulfonic acid (PFNS) | 5.239 | 2.5 | 549 | 80 | 99 | – | – |
| 48. | N-methyl-d 3-perfluoro-1-octanesulfonamidoacetic acid (D3-NMeFOSAA) | 5.361 | 5 | 573 | 419 | – | – | – |
| 49. | N-methyl perfluorooctanesulfonamidoacetic acid (NMeFOSAA) | 5.366 | 2.5 | 570 | 419 | 483 | – | – |
| 50. | Perfluorodecanesulfonic acid (PFDS) | 5.475 | 2.5 | 599 | 80 | 99 | – | – |
| 51. | Perfluoroundecanoic acid (PFUnA) | 5.486 | 2.5 | 563 | 519 | 269 | – | – |
| 52. | Perfluoro-n-[1,2,3,4,5,6,7- 13C7]undecanoic acid (13C7-PFUnA) | 5.486 | 1.25 | 570 | 525 | – | – | – |
| 53. | N-ethyl-d 5-perfluoro-1-octanesulfonamidoacetic acid (D5-NEtFOSAA) | 5.487 | 5 | 589 | 419 | – | – | – |
| 54. | N-ethyl perfluorooctanesulfonamidoacetic acid (NEtFOSAA) | 5.487 | 2.5 | 584 | 419 | 526 | – | – |
| 55. | 11-Chloroeicosafluoro-3-oxaundecane-1-sulfonic acid (11Cl-PF3OUdS) | 5.59 | 10 | 631 | 451 | – | 633 | 453 |
| 56. | Perfluorooctanesulfonamide (PFOSA) | 5.669 | 2.5 | 498 | 78 | 478 | – | – |
| 57. | Perfluoro-1-[ 13C8]octanesulfonamide (13C8-PFOSA) | 5.669 | 2.5 | 506 | 78 | – | – | – |
| 58. | Perfluorododecanoic acid (PFDoA) | 5.691 | 2.5 | 613 | 569 | 319 | – | – |
| 59. | Perfluoro-n-[1,2- 13C2]dodecanoic acid (13C2-PFDoA) | 5.692 | 1.25 | 615 | 570 | – | – | – |
| 60. | Perfluorododecanesulfonic acid (PFDoS) | 5.861 | 2.5 | 699 | 80 | 99 | – | – |
| 61. | Perfluorotridecanoic acid (PFTrDA) | 5.876 | 2.5 | 663 | 619 | 169 | – | – |
| 62. | Perfluorotetradecanoic acid (PFTeDA) | 6.031 | 2.5 | 713 | 669 | 169 | – | – |
| 63. | Perfluoro-n-[1,2- 13C2]tetradecanoic acid (13C2-PFTeDA) | 6.031 | 1.25 | 715 | 670 | – | – | – |
| 64. | N-methyl perfluorooctanesulfonamide (NMeFOSA) | 6.158 | 2.5 | 512 | 219 | 169 | – | – |
| 65. | N-methyl-D 3-perfluoro-1-octanesulfonamide (D3-NMeFOSA) | 6.159 | 2.5 | 515 | 219 | – | – | – |
| 66. | N-methyl perfluorooctanesulfonamidoethanol (NMeFOSE) | 6.166 | 25 | 616 | 59 | – | – | – |
| 67. | N-methyl-D 7-perfluorooctanesulfonamidoethanol (D7-NMeFOSE) | 6.167 | 25 | 623 | 59 | – | – | – |
| 68. | N-ethyl-D 5-perfluoro-1-octanesulfonamide (D5-NEtFOSA) | 6.295 | 2.5 | 531 | 219 | – | – | – |
| 69. | N-ethyl perfluorooctanesulfonamide (NEtFOSA) | 6.296 | 2.5 | 526 | 219 | 169 | – | – |
| 70. | N-ethyl perfluorooctanesulfonamidoethanol (NEtFOSE) | 6.298 | 25 | 630 | 59 | – | – | – |
| 71. | N-ethyl-D 9-perfluorooctanesulfonamidoethanol (D9-NEtFOSE) | 6.298 | 25 | 639 | 59 | – | – | – |
Conditions
| Column | Force C18 (cat.# 9634252) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 50 mm x 2.1 mm ID | ||||||||||||||||||||||||
| Particle Size: | 1.8 µm | ||||||||||||||||||||||||
| Pore Size: | 100 Å | ||||||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||
| Inj. Vol.: | 3 µL | ||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||
| A: | Water, 5 mM ammonium acetate | ||||||||||||||||||||||||
| B: | Methanol | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Max Pressure: | ~400 bar |
| Detector | Waters Xevo TQ-S |
|---|---|
| Ion Mode: | ESI- |
| Mode: | MRM |
| Instrument | Waters ACQUITY Premier |
| Sample Preparation | Resprep PFAS cartridges (cat.# 28930) and a Thermo AutoTrace PFAS instrument were used for the following sample preparation procedure. 1. Spike 500 mL DI water with 200 µL of Wellington EPA-1633STK and 25 µL of Wellington MPFAC-HIF-ES. 2. Rinse cartridge with 15 mL of 1% ammonium hydroxide in methanol. 3. Rinse with 5 mL of 0.3 M formic acid in water. 4. Load sample onto SPE cartridge dropwise at 5 mL/min. 5. Rinse bottle with 5 mL DI water, load onto SPE cartridge dropwise at 5 mL/min, repeat. 6. Rinse bottle with 5 mL of 1:1 0.1 M formic acid:methanol and load onto SPE cartridge dropwise at 5 mL/min. 7. Flow nitrogen through cartridge for 15 minutes. 8. Rinse bottles with 5 mL of 1% ammonium hydroxide in methanol, elute into collection vessel. 9. Add 25 µL of concentrated acetic acid and 25 µL of Wellington MPFAC-HIF-IS. 10. Transfer aliquot to autosampler vial (cat. # 23243) and cap (cat. # 23244). |
| Notes | A PFAS delay column (cat.# 27854) was installed before the injector. |
Table I: Results from MDL, Blank, Precision, and Accuracy Experiments for Native PFAS
| Compound | Abbreviation | MDL (ng/L) | Blank (ng/L)* | Accuracy (%) | %RSD |
| Perfluorobutanoic acid | PFBA | 0.34 | ND | 112 | 5 |
| Perfluoro-3-methoxypropanoic acid | PFMPA | 0.20 | ND | 109 | 6 |
| 3-Perfluoropropyl propanoic acid | 3:3FTCA | 0.31 | ND | 95 | 6 |
| Perfluoropentanoic acid | PFPeA | 0.26 | ND | 111 | 6 |
| Perfluorobutane sulfonate | PFBS | 0.19 | ND | 101 | 5 |
| Perfluoro-4-methoxybutanoic acid | PFMBA | 0.18 | ND | 111 | 6 |
| Perfluoro(2-ethoxyethane)sulfonic acid | PFEESA | 0.15 | ND | 94 | 5 |
| Nonafluoro-3,6-dioxaheptanoic acid | NFDHA | 0.46 | ND | 121 | 5 |
| 1H,1H,2H,2H-perfluorohexane sulfonate | 4:2 FTS | 0.36 | ND | 104 | 5 |
| 2H,2H,3H,3H-Perfluorooctanoic acid | 5:3FTCA | 1.48 | ND | 102 | 6 |
| Perfluorohexanoic acid | PFHxA | 0.08 | ND | 113 | 5 |
| Perfluoropentane sulfonate | PFPeS | 0.07 | ND | 122 | 4 |
| Hexafluoropropylene oxide dimer acid | HFPO-DA | 0.40 | ND | 114 | 12 |
| Perfluoroheptanoic acid | PFHpA | 0.08 | ND | 109 | 5 |
| Perfluorohexane sulfonate | PFHxS | 0.07 | ND | 104 | 7 |
| 4,8-Dioxa-3H-perfluorononanoic acid | ADONA | 0.39 | ND | 99 | 9 |
| 1H,1H,2H,2H-perfluorooctane sulfonate | 6:2 FTS | 0.37 | ND | 107 | 4 |
| 3-Perfluoroheptyl propanoic acid | 7:3FTCA | 2.32 | ND | 100 | 6 |
| Perfluoroheptane sulfonate | PFHpS | 0.76 | ND | 121 | 8 |
| Perfluorooctanoic acid | PFOA | 0.32 | ND | 104 | 2 |
| Perfluorooctane sulfonate | PFOS | 0.20 | ND | 112 | 7 |
| Perfluorooctanesulfonamide | PFOSA | 0.21 | ND | 81 | 11 |
| Perfluorononanoic acid | PFNA | 0.08 | ND | 114 | 5 |
| N-methyl perfluorooctanesulfonamide | NMeFOSA | 0.07 | ND | 104 | 13 |
| 9-Chlorohexadecafluoro-3-oxanonane-1-sulfonic acid | 9Cl-PF3ONS | 0.61 | ND | 85 | 11 |
| Perfluorononanesulfonic acid | PFNS | 0.25 | ND | 79 | 7 |
| Perfluorodecanoic acid | PFDA | 0.18 | ND | 117 | 8 |
| N-ethyl perfluorooctanesulfonamide | NEtFOSA | 0.14 | ND | 112 | 15 |
| 1H,1H,2H,2H-perfluorodecane sulfonate | 8:2 FTS | 0.91 | ND | 113 | 11 |
| Perfluoroundecanoic acid | PFUnA | 0.26 | ND | 125 | 12 |
| N-methyl perfluorooctanesulfonamidoacetic acid | NMeFOSAA | 0.15 | ND | 95 | 12 |
| N-ethyl perfluorooctanesulfonamidoacetic acid | NEtFOSAA | 0.23 | ND | 93 | 12 |
| Perfluorodecanesulfonic acid | PFDS | 0.25 | ND | 94 | 23 |
| 11-Chloroeicosafluoro-3-oxaundecane-1-sulfonic acid | 11Cl-PF3OUdS | 0.56 | ND | 77 | 25 |
| Perfluorododecanoic acid | PFDoA | 0.27 | ND | 118 | 15 |
| N-methyl perfluorooctanesulfonamidoethanol | NMeFOSE | 1.11 | ND | 106 | 9 |
| N-ethyl perfluorooctanesulfonamidoethanol | NEtFOSE | 1.10 | ND | 111 | 9 |
| Perfluorotridecanoic acid | PFTrDA | 0.33 | ND | 108 | 15 |
| Perfluorododecanesulfonic acid | PFDoS | 0.13 | ND | 89 | 12 |
| Perfluorotetradecanoic acid | PFTeDA | 0.32 | ND | 102 | 23 |
*ND = Not detected above MDL
Table II: Results from Precision and Accuracy Experiments for Isotope Dilution Standards
| Compound | Abbreviation | Accuracy (%) | %RSD |
| Perfluoro-n-[1,2,3,4- 13C4]butanoic acid | 13C4-PFBA | 88 | 4 |
| Perfluoro-n-[1,2,3,4,5- 13C5]pentanoic acid | 13C5-PFPeA | 88 | 5 |
| Sodium perfluoro-1-[2,3,4- 13C3]butanesulfonate | 13C3-PFBS | 97 | 3 |
| Sodium 1H,1H,2H,2H-perfluoro-1-[1,2- 13C2]hexane sulfonate | 13C2-4:2FTS | 95 | 8 |
| Perfluoro-n-[1,2,3,4,6- 13C5]hexanoic acid | 13C5-PFHxA | 122 | 2 |
| 2,3,3,3-Tetrafluoro-2-(1,1,2,2,3,3,3-heptafluoropropoxy13C3-propanoic acid | 13C3-HFPO-DA | 92 | 4 |
| Perfluoro-n-[1,2,3,4- 13C4]heptanoic acid | 13C4-PFHpA | 107 | 4 |
| Sodium perfluoro-1-[1,2,3- 13C3]hexanesulfonate | 13C3-PFHxS | 102 | 4 |
| Sodium 1H,1H,2H,2H-perfluoro-1-[1,2-13C2]-octane sulfonate | 13C2-6:2FTS | 83 | 5 |
| Perfluoro-n-[ 13C8]octanoic acid | 13C8-PFOA | 106 | 5 |
| Sodium perfluoro-[ 13C8]octanesulfonate | 13C8-PFOS | 77 | 7 |
| Perfluoro-1-[ 13C8]octanesulfonamide | 13C8-PFOSA | 94 | 11 |
| N-methyl-d 3-perfluoro-1-octanesulfonamidoacetic acid | D3-NMeFOSAA | 85 | 9 |
| N-ethyl-d 5-perfluoro-1-octanesulfonamidoacetic acid | D5-NEtFOSAA | 87 | 11 |
| Perfluoro-n-[ 13C9]nonanoic acid | 13C9-PFNA | 83 | 7 |
| Perfluoro-n-[1,2,3,4,5,6- 13C6]decanoic acid | 13C6-PFDA | 80 | 7 |
| Sodium 1H,1H,2H,2H-perfluoro-1-[1,2- 13C2]-decane sulfonate | 13C2-8:2FTS | 84 | 5 |
| N-methyl-D 7-perfluorooctanesulfonamidoethanol | D7-NMeFOSE | 108 | 13 |
| N-ethyl-D 9-perfluorooctanesulfonamidoethanol | D9-NEtFOSE | 88 | 17 |
| N-methyl-D 3-perfluoro-1-octanesulfonamide | D3-NMeFOSA | 84 | 8 |
| N-ethyl-D 5-perfluoro-1-octanesulfonamide | D5-NEtFOSA | 111 | 10 |
| Perfluoro-n-[1,2,3,4,5,6,7- 13C7]undecanoic acid | 13C7-PFUnA | 78 | 13 |
| Perfluoro-n-[1,2- 13C2]dodecanoic acid | 13C2-PFDoA | 45 | 24 |
| Perfluoro-n-[1,2- 13C2]tetradecanoic acid | 13C2-PFTeDA | 36 | 21 |
Filtration and Sample Flow Experiment
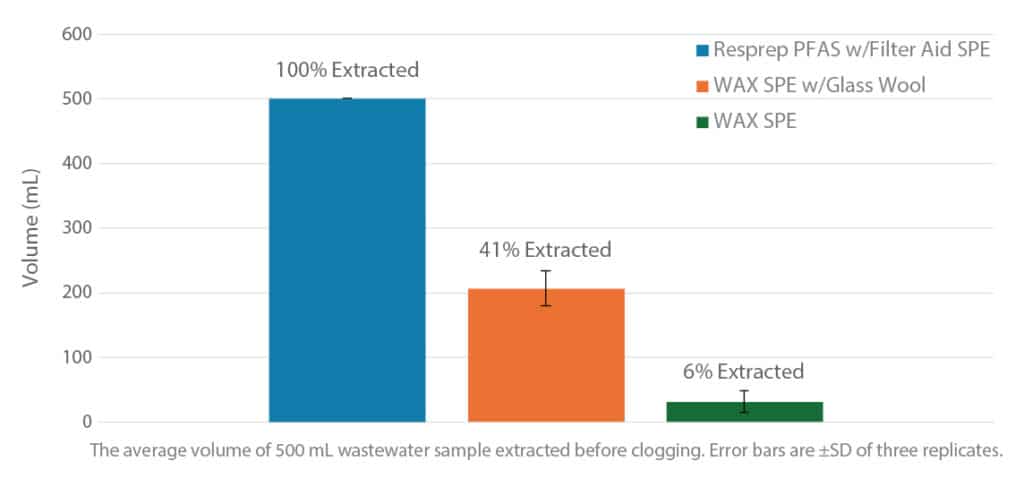
Resprep PFAS SPE cartridges are available with and without a factory-packed filter aid that allows faster, more consistent sample preparation compared to using manually packed glass wool. As demonstrated in Figure 3, the filter aid provides another significant benefit: it prevents clogging so that the full sample can be processed, saving the time, effort, and cost of a second extraction. In this comparison, only the Resprep PFAS cartridge with filter aid allowed for extraction of the full 500 mL of the substitute wastewater matrix containing 100 mg/L suspended solids. This is a great improvement over the use of glass wool, which could extract less than half of the sample, and WAX alone, which could extract less than a tenth of the sample.
Conclusions
Nondrinking water matrices present unique challenges to labs using SPE sample preparation. Resprep PFAS cartridges allows labs to handle difficult water matrices more efficiently while meeting or exceeding the requirements of EPA Method 1633 for PFAS analysis. Visit www.restek.com/PFAS for additional products, methods, and technical resources.
References
- U.S. Environmental Protection Agency, Method 1633, Analysis of per- and polyfluoroalkyl substances (PFAS) in aqueous, solid, biosolids, and tissue samples by LC-MS/MS, January 2024. https://www.epa.gov/system/files/documents/2024-01/method-1633-final-for-web-posting.pdf
- ASTM International, D5905-98(2018), Standard practice for the preparation of substitute wastewater, December 2018. https://www.astm.org/d5905-98r18.html
- U.S. Environmental Protection Agency, Definition and procedure for the determination of the method detection limit, Revision 2, December 2016. https://www.epa.gov/sites/default/files/2016-12/documents/mdl-procedure_rev2_12-13-2016.pdf





