Key Highlights
- Highly effective GC column deactivation produces an exceptionally inert sample flow path.
- Maximum inertness results in sharp, symmetrical peaks and single or sub-picogram instrument detection limits (IDL) for a wide range of challenging semivolatiles.
- Data quality objectives for calibration were easily met with ≤20% relative standard error (RSE) for all semivolatiles and lowest calibration points of 1-10 ppb for all compounds, except benzoic acid (50 ppb).

Abstract
This application note evaluates the performance of an RMX-5Sil MS column for trace-level GC-MS/MS analysis of semivolatile organic compounds. Single or sub-picogram instrument detection limits were achieved for all compounds, except benzoic acid (14.70 pg) and 2,4-dinitrophenol (11.53 pg). Calibration curve %RSE was ≤20% for all compounds, and curves ranged from 1-1000 ppb to 10-1000 ppb for all compounds, except benzoic acid, which was 50-1000 ppb.
Introduction
Environmental testing laboratories routinely analyze semivolatile organic compounds (SVOCs) by GC-MS or GC-MS/MS using methods such as EPA Method 8270E. Labs are increasingly adopting GC-MS/MS methods because the improved selectivity of the detector allows for more sensitive analyses, such as those needed for EPA Method 3511 microextraction, which saves time and reduces solvent consumption compared to traditional sample preparation methods that can use up to a liter of sample. To achieve the full benefits of MS/MS sensitivity, the analytical column must be highly inert to maximize peak signal-to-noise ratios. A broadly effective column deactivation is essential for ensuring good overall method performance across a wide range of compound chemistries that interact via different mechanisms with any active sites present on the column surface. In this study, an RMX-5Sil MS column was independently evaluated for analytical performance across a wide range of trace-level semivolatiles, including compounds that are known to be very challenging. Chromatographic performance and calibration ranges were assessed to evaluate the column’s suitability for achieving picogram-level detection.
Experimental
Standard Preparation
Multicomponent calibration standards were prepared in methylene chloride across a range of 1-1000 ppb (11 points) from commercially available reference standards.
Instrument Conditions
Samples were run on an RMX-5Sil MS column in a 30 m, 0.25 mm ID, 0.25 μm format (cat.# 17323). A Shimadzu Nexis GC-2030 GC paired with a Shimadzu GCMS-TQ8050 NX ultra-fast mass spectrometer with EI source and UFsweeper high-efficiency collision cell was used for GC-MS/MS semivolatiles analysis under the conditions listed below.
Table I: GC-MS/MS Method Conditions for Trace-Level Semivolatiles Analysis
| Gas Chromatograph | Shimadzu Nexis GC-2030 |
| Column | RMX-5Sil MS, 30 m x 0.25 mm ID x 0.25 µm (cat.# 17323) |
| Flow mode | Constant linear velocity (39.5 cm/s) |
| Injector mode | Split (5:1) |
| Injector liner | Topaz 3.5 mm ID single taper inlet liner w/wool (cat.# 23336) |
| Injection volume | 1 µL |
| Injector temperature | 275 °C |
| Oven program | 40 °C (hold 1.5 min) to 280 °C at 20 °C /min to 320 °C at 5 °C /min (hold 1 min) |
| MS system | Shimadzu GCMS-TQ8050 NX ultra-fast mass spectrometer with EI source and UFsweeper high-efficiency collision cell |
| Ionization | Electron impact |
| Ionization energy | 70 eV |
| Emission current | 60 mA |
| Acquisition mode | MRM |
| Collision gas | Argon |
| Source temperature | 230 °C |
| GC interface temperature | 300 °C |
Results and Discussion
Chromatographic Performance
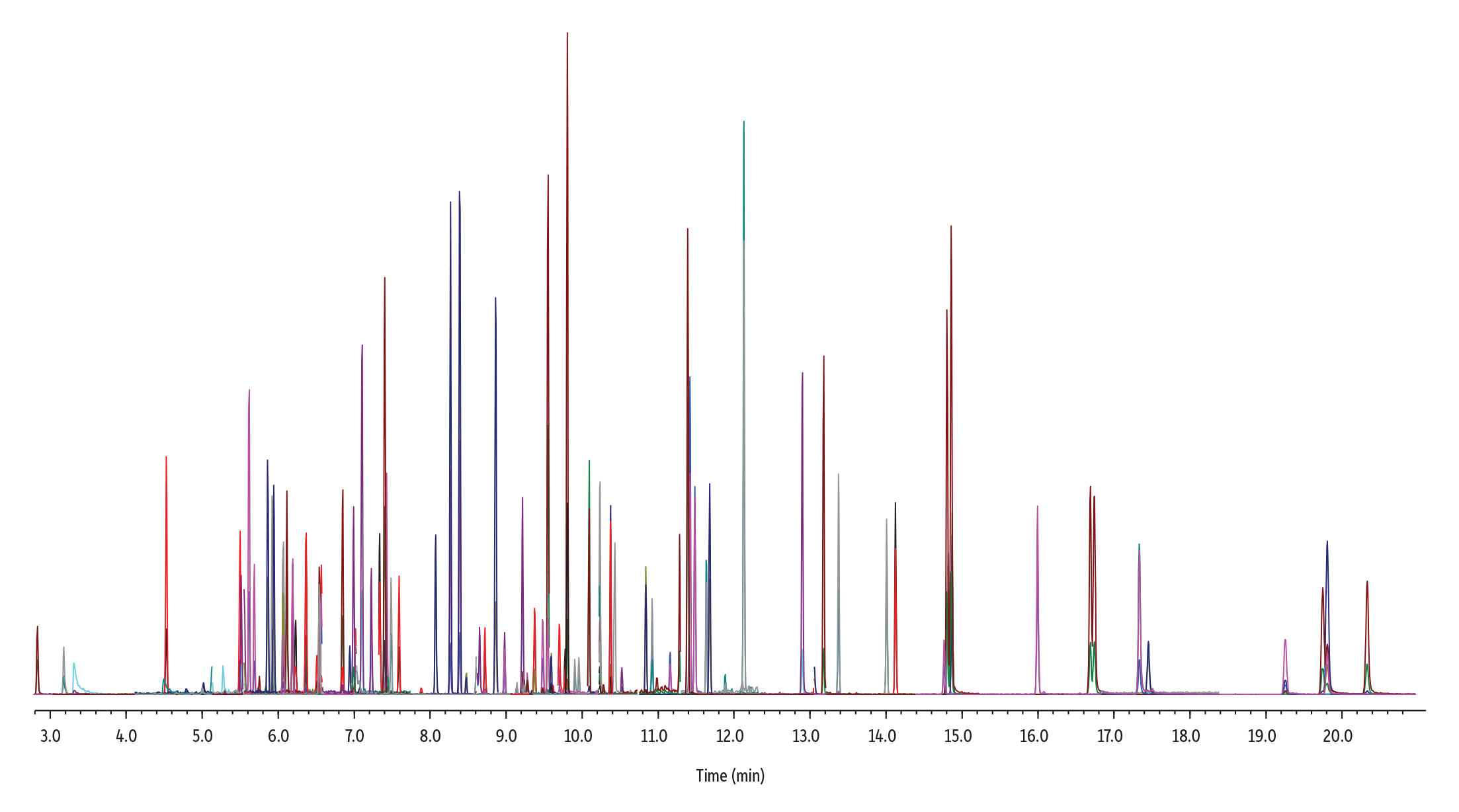
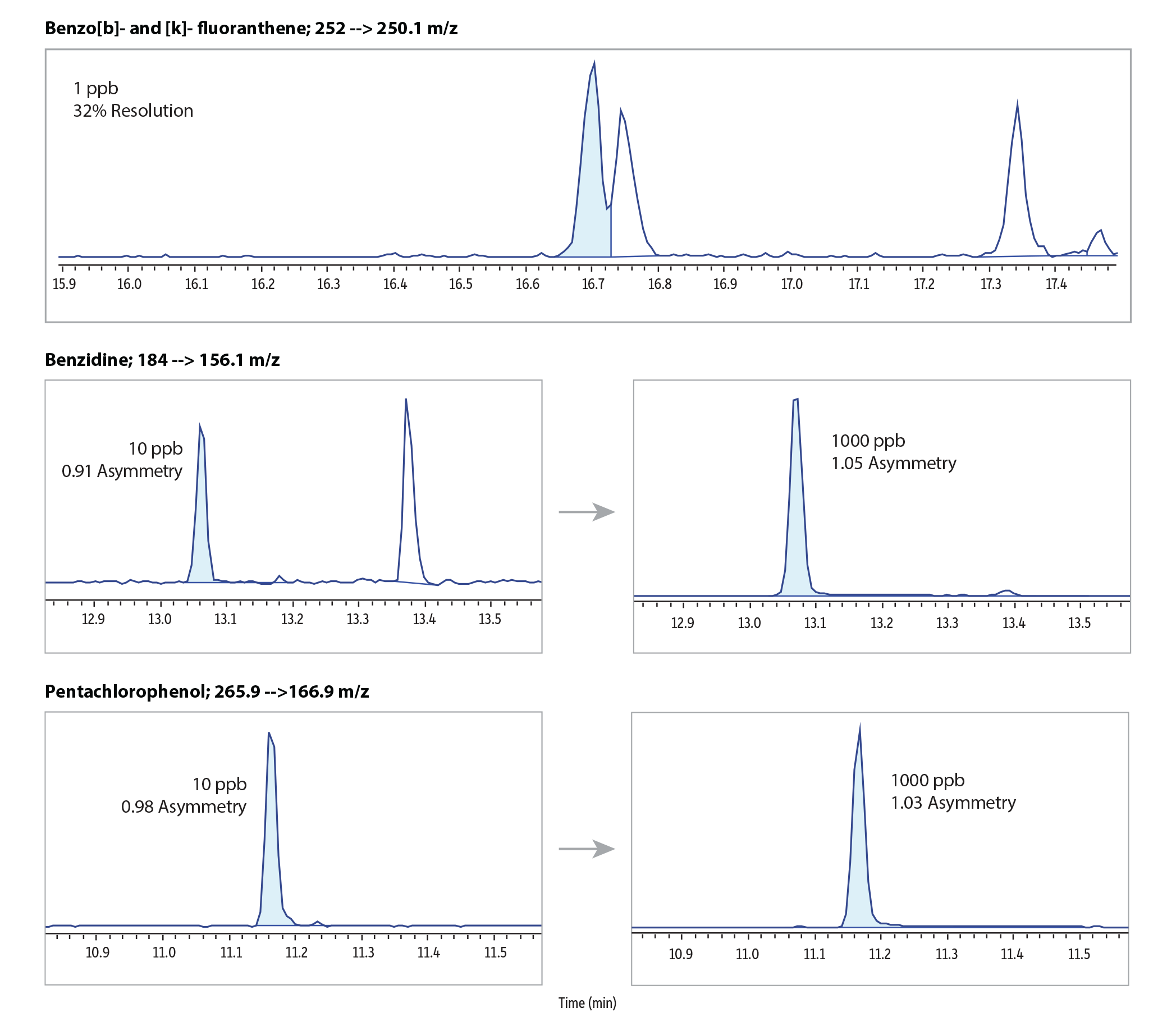
Overall, the RMX-5Sil MS column produced excellent peak shapes and separations for 86 semivolatiles at 50 ppb across the chromatographic space with the last compound eluting in just under 21 minutes (Figure 1). To evaluate column inertness, peak tailing was measured at or near the low and high ends of the linear calibration range for acidic and basic compounds that are known to be particularly problematic. As shown in Figure 2, the inert surface minimized surface activity at low levels and maximized peak symmetry for easy, accurate integration, even when difficult compounds were being integrated at low concentrations. In addition, the surface did not show any negative effects on polymer selectivity, as demonstrated by good separation of the closely eluting polycyclic aromatic hydrocarbon (PAH) pair, benzo(b)fluoranthene and benzo(k)fluoranthene at 1 ppb.
Figure 1: GC-MS/MS Analysis of 86 Semivolatiles on an RMX-5Sil MS Column (50 ppb, TIC)
GC_EV1534
Peaks
| Peaks | tR (min) | Transition 1 | Collision energy 1 | Transition 2 | Collision energy 2 | |
|---|---|---|---|---|---|---|
| 1. | N-Nitrosodimethylamine | 3.173 | 74.00>44.10 | 6 | 74.00>42.10 | 18 |
| 2. | Pyridine | 3.276 | 79.10>50.10 | 21 | 79.10>52.10 | 15 |
| 3. | 2-Fluorophenol | 4.532 | 112.00>64.10 | 18 | 112.00>92.10 | 9 |
| 4. | Phenol | 5.514 | 94.00>66.00 | 9 | 66.00>40.00 | 12 |
| 5. | Phenol-d5 Surr | 5.5 | 99.00>71.10 | 12 | 99.00>69.10 | 27 |
| 6. | Aniline | 5.554 | 93.00>66.10 | 18 | 93.00>51.10 | 30 |
| 7. | Bis(2-chloroethyl) ether | 5.619 | 93.00>63.10 | 9 | 95.00>65.00 | 6 |
| 8. | 2-Chlorophenol | 5.688 | 128.00>64.00 | 18 | 128.00>91.90 | 15 |
| 9. | 1,3-Dichlorobenzene | 5.863 | 146.00>111.10 | 21 | 146.00>75.20 | 30 |
| 10. | 1,4-Dichlorobenzene | 5.944 | 146.00>111.10 | 21 | 146.00>75.20 | 30 |
| 11. | Benzyl alcohol | 6.069 | 79.00>77.10 | 12 | 107.00>79.10 | 9 |
| 12. | 1,2-Dichlorobenzene | 6.116 | 146.00>111.10 | 21 | 146.00>75.20 | 30 |
| 13. | 2-Methylphenol | 6.194 | 108.00>77.00 | 27 | 108.00>79.00 | 18 |
| 14. | 2,2′-oxybis(1-chloropropane) | 6.229 | 121.05>77.00 | 9 | 121.05>45.00 | 6 |
| 15. | 3 and 4-Methylphenol | 6.369 | 107.10>77.10 | 15 | 107.10>79.10 | 6 |
| 16. | N-Nitrosodi-N-propylamine | 6.374 | 130.10>113.10 | 4 | 130.10>88.10 | 4 |
| 17. | Hexachloroethane | 6.511 | 117.00>81.90 | 30 | 119.00>83.80 | 33 |
| 18. | Nitrobenzene | 6.563 | 77.05>51.00 | 21 | 123.05>77.00 | 15 |
| 19. | Nitrobenzene-d5 | 6.541 | 82.00>54.10 | 18 | 128.00>82.10 | 18 |
| 20. | Isophorone | 6.846 | 82.00>54.00 | 9 | 138.00>82.00 | 18 |
| 21. | 2-Nitrophenol | 6.941 | 139.00>109.10 | 9 | 139.00>81.00 | 12 |
| 22. | 2,4-Dimethylphenol | 6.99 | 107.00>77.10 | 18 | 122.00>107.10 | 18 |
| 23. | Benzoic Acid | 7.019 | 122.10>105.10 | 9 | 105.10>77.10 | 15 |
| 24. | Bis(2-chloroethoxy)methane | 7.102 | 93.00>63.10 | 9 | 95.00>65.00 | 6 |
| 25. | 2,4-Dichlorophenol | 7.223 | 162.00>63.10 | 33 | 164.00>63.10 | 30 |
| 26. | 1,2,4-Trichlorobenzene | 7.333 | 180.00>109.00 | 30 | 180.00>145.10 | 18 |
| 27. | Naphthalene | 7.424 | 128.10>102.10 | 20 | 128.10>78.00 | 20 |
| 28. | 2,6-Dichlorophenol | 7.223 | 136.00>108.10 | 27 | 136.00>134.10 | 27 |
| 29. | 4-Chloroaniline | 7.484 | 127.00>65.10 | 27 | 127.00>92.10 | 18 |
| 30. | Hexachlorobutadiene | 7.59 | 225.00>189.80 | 21 | 225.00>155.00 | 30 |
| 31. | 4-Chloro-3-methylphenol | 8.072 | 107.00>77.10 | 18 | 142.00>107.00 | 18 |
| 32. | 2-Methylnaphthalene | 8.268 | 142.10>115.10 | 28 | 115.10>89.00 | 16 |
| 33. | 1-Methylnaphthalene | 8.388 | 142.10>115.10 | 28 | 115.10>89.00 | 16 |
| 34. | Hexachlorocyclopentadiene | 8.474 | 237.00>141.00 | 27 | 237.00>143.00 | 27 |
| 35. | 2,4,6-Trichlorophenol | 8.613 | 196.00>97.00 | 33 | 198.00>97.00 | 30 |
| 36. | 2,4,5-Trichlorophenol | 8.651 | 196.00>97.00 | 33 | 198.00>97.00 | 30 |
| 37. | 2-Fluorobiphenyl | 8.721 | 172.00>151.20 | 27 | 172.00>146.10 | 27 |
| 38. | 2-Chloronaphthalene | 8.862 | 162.00>127.10 | 18 | 162.00>77.10 | 33 |
| 39. | 2-Nitroaniline | 8.979 | 138.00>92.00 | 15 | 138.00>65.10 | 33 |
| 40. | 1,4-Dinitrobenzene | 9.143 | 168.00>75.10 | 30 | 168.00>92.00 | 15 |
| 41. | Dimethylphthalate | 9.216 | 163.00>77.20 | 15 | 163.00>133.10 | 15 |
| 42. | 1,3-Dinitrobenzene | 9.14 | 168.00>75.00 | 30 | 168.00>122.00 | 12 |
| 43. | 2,6-Dinitrotoluene | 9.281 | 165.00>90.00 | 15 | 165.00>63.10 | 33 |
| 44. | Acenapthylene | 9.374 | 152.10>150.10 | 28 | 152.10>126.10 | 28 |
| 45. | 3-Nitroaniline | 9.484 | 92.05>65.00 | 12 | 138.05>65.00 | 27 |
| 46. | Acenaphthene | 9.592 | 152.10>150.10 | 28 | 152.10>126.10 | 28 |
| 47. | 2,4-Dinitrophenol | 9.617 | 184.05>107.00 | 12 | 154.05>107.00 | 6 |
| 48. | 4-Nitrophenol | 9.689 | 109.05>81.00 | 12 | 109.05>53.10 | 18 |
| 49. | 2,4-Dinitrotoluene | 9.778 | 89.05>63.10 | 18 | 165.05>119.00 | 6 |
| 50. | Dibenzofuran | 9.805 | 168.00>139.10 | 24 | 139.00>89.10 | 21 |
| 51. | 2,3,5,6-Tetrachlorophenol | 9.904 | 230.00>130.90 | 36 | 232.00>132.90 | 36 |
| 52. | 2,3,4,6-Tetrachlorophenol | 9.958 | 230.00>130.90 | 36 | 232.00>132.90 | 36 |
| 53. | Diethylphthalate | 10.093 | 149.00>65.00 | 30 | 177.00>149.10 | 12 |
| 54. | 4-Chlorophenyl phenyl ether | 10.234 | 141.00>115.20 | 21 | 204.00>141.20 | 21 |
| 55. | Fluorene | 10.23 | 165.10>163.10 | 28 | 165.10>115.10 | 28 |
| 56. | 4-Nitroaniline | 10.234 | 138.00>108.10 | 12 | 108.00>80.00 | 12 |
| 57. | 4,6-Dinitro-2-methylphenol | 10.283 | 198.05>121.00 | 12 | 198.05>53.00 | 27 |
| 58. | N-Nitrosodiphenylamine (Diphenylamine) | 10.376 | 169.00>167.20 | 27 | 168.00>139.00 | 39 |
| 59. | Diphenylhydrazine | 10.429 | 77.00>51.20 | 15 | 77.00>74.10 | 33 |
| 60. | 2,4,6-Tribromophenol | 10.524 | 329.80>141.00 | 36 | 331.80>142.90 | 36 |
| 61. | 4-Bromophenyl phenyl ether | 10.839 | 250.00>141.10 | 21 | 248.00>141.10 | 18 |
| 62. | Hexachlorobenzene | 10.922 | 283.80>248.80 | 24 | 283.80>213.80 | 28 |
| 63. | Pentachlorophenol | 11.159 | 265.90>164.90 | 26 | 265.90>166.90 | 26 |
| 64. | Phenanthrene | 11.421 | 178.10>176.10 | 28 | 178.10>152.10 | 20 |
| 65. | Anthracene | 11.484 | 178.10>176.10 | 28 | 178.10>152.10 | 20 |
| 66. | Carbazole | 11.68 | 167.00>139.20 | 27 | 166.00>140.00 | 18 |
| 67. | Di-n-butylphthalate | 12.131 | 149.00>93.10 | 18 | 149.00>65.10 | 24 |
| 68. | Fluoranthene | 12.9 | 202.10>200.10 | 30 | 200.10>198.10 | 30 |
| 69. | Benzidine | 13.062 | 184.00>156.10 | 24 | 184.00>167.10 | 24 |
| 70. | Pyrene | 13.18 | 202.10>200.10 | 30 | 200.10>198.10 | 30 |
| 71. | o-Terphenyl-D14 | 13.377 | 244.00>240.10 | 30 | 244.00>226.20 | 18 |
| 72. | Butylbenzylphthalate | 14.012 | 149.00>65.10 | 24 | 149.00>93.10 | 18 |
| 73. | Bis(2-ethylhexyl)adipate | 14.129 | 129.00>55.10 | 21 | 129.00>101.10 | 9 |
| 74. | 3’3-Dichlorobenzidine | 14.769 | 212.00>180.10 | 24 | 212.00>196.20 | 21 |
| 75. | Benz[a]anthracene | 14.805 | 228.10>226.10 | 32 | 226.10>224.10 | 32 |
| 76. | Bis(2-ethylhexyl)phthalate | 14.875 | 149.00>65.10 | 24 | 167.00>149.10 | 15 |
| 77. | Chrysene | 14.861 | 228.10>226.10 | 32 | 226.10>224.10 | 32 |
| 78. | Di-n-octylphthalate | 16 | 149.00>65.10 | 24 | 149.00>93.20 | 18 |
| 79. | Benzo[b]fluoranthene | 16.691 | 252.10>250.10 | 36 | 250.10>248.10 | 36 |
| 80. | Benzo[k]fluoranthene | 16.746 | 252.10>250.10 | 36 | 250.10>248.10 | 36 |
| 81. | Benzo[a]pyrene | 17.338 | 252.10>250.10 | 36 | 250.10>248.10 | 36 |
| 82. | Dibenz[a,j]acridine | 19.26 | 279.00>277.10 | 33 | 279.00>250.00 | 45 |
| 83. | Indeno[1,2,3-cd]pyrene | 19.754 | 276.10>274.10 | 36 | 274.10>272.10 | 36 |
| 84. | Dibenz[a,h]anthracene | 19.813 | 278.10>276.10 | 36 | 278.10>274.10 | 60 |
| 85. | Benzo[g,h,i]perylene | 20.339 | 276.10>274.10 | 36 | 274.10>272.10 | 36 |
Conditions
| Column | RMX-5Sil MS, 30 m, 0.25 mm ID, 0.25 µm (cat.# 17323) |
|---|---|
| Injection | |
| Inj. Vol.: | 1 µL split (split ratio 5:1) |
| Liner: | Topaz 3.5 mm ID single taper inlet liner w/wool (cat.# 23336) |
| Inj. Temp.: | 275 °C |
| Oven | |
| Oven Temp.: | 40 °C (hold 1.5 min) to 280 °C at 20 °C/min to 320 °C at 5 °C/min (hold 1 min) |
| Carrier Gas | He, constant flow |
| Linear Velocity: | 39.5 cm/sec @ 40 °C |
| Detector | SRM/MRM |
|---|---|
| Acquisition Type: | SRM/MRM |
| Source Temp.: | 230 °C |
| Transfer Line Temp.: | 300 °C |
| Analyzer Type: | Triple Quadrupole |
| Ionization Mode: | EI |
| Collision Gas: | Ar |
| Tune Type: | PFTBA |
| Tune Emission Current: | 60 μA |
| Notes | Shimadzu Nexis GC-2030 with Shimadzu GCMS-TQ8050 NX ultra-fast mass spectrometer with EI source and UFsweeper high-efficiency collision cell |
| Acknowledgement | Shimadzu |
Figure 2: The highly effective deactivation used in RMX-5Sil MS columns creates an exceptionally inert surface that produces symmetrical peaks for a wide range of semivolatiles, including acidic compounds (pentachlorophenol); basic compounds (benzidine); and closely eluting PAHs.
GC_EV1535
Peaks
| Peaks | tR (min) | Transition 1 | Collision energy 1 | Transition 2 | Collision energy 2 | |
|---|---|---|---|---|---|---|
| 1. | Benzidine | 13.062 | 184.00>156.10 | 24 | 184.00>167.10 | 24 |
| 2. | Benzo[b]fluoranthene | 16.691 | 252.10>250.10 | 36 | 250.10>248.10 | 36 |
| 3. | Benzo[k]fluoranthene | 16.746 | 252.10>250.10 | 36 | 250.10>248.10 | 36 |
| 4. | Pentachlorophenol | 11.159 | 265.90>164.90 | 26 | 265.90>166.90 | 26 |
Conditions
| Column | RMX-5Sil MS, 30 m, 0.25 mm ID, 0.25 µm (cat.# 17323) |
|---|---|
| Injection | |
| Inj. Vol.: | 1 µL split (split ratio 5:1) |
| Liner: | Topaz 3.5 mm ID single taper inlet liner w/wool (cat.# 23336) |
| Inj. Temp.: | 275 °C |
| Oven | |
| Oven Temp.: | 40 °C (hold 1.5 min) to 280 °C at 20 °C/min to 320 °C at 5 °C/min (hold 1 min) |
| Carrier Gas | He, constant flow |
| Linear Velocity: | 39.5 cm/sec @ 40 °C |
| Detector | SRM/MRM |
|---|---|
| Acquisition Type: | SRM/MRM |
| Source Temp.: | 230 °C |
| Transfer Line Temp.: | 300 °C |
| Analyzer Type: | Triple Quadrupole |
| Ionization Mode: | EI |
| Collision Gas: | Ar |
| Tune Type: | PFTBA |
| Tune Emission Current: | 60 μA |
| Notes | Shimadzu Nexis GC-2030 with Shimadzu GCMS-TQ8050 NX ultra-fast mass spectrometer with EI source and UFsweeper high-efficiency collision cell |
| Acknowledgement | Shimadzu |

Calibration Performance
As demonstrated in Table II, the combination of the Shimadzu GC-MS/MS system and the highly inert RMX-5Sil MS analytical column allowed for outstanding trace-level sensitivity for a broad range of semivolatile compound classes. The calibration curve %RSE was ≤20% for all compounds, which met data quality objectives for calibration. In addition, R2 was determined for a subset of representative semivolatiles and was found to be ≥0.99 for all compounds that were assessed. For all semivolatiles, the calibration curves ranged from 1-1000 ppb to 10-1000 ppb, except benzoic acid which was 50-1000 ppb. A slightly higher linear range for benzoic acid was not surprising because it does not solubilize well in 5-type phases. Finally, very low instrument detection limits were achieved and were single or sub-picogram levels for all semivolatiles, except benzoic acid (14.70 pg) and 2,4-dinitrophenol (11.53 pg).
Table II: Calibration Performance for Trace-Level Semivolatiles Analysis on an RMX-5Sil MS Column
| Compound Name | Retention Time (min) | Calibration Curve %RSE | R2 | Low Point (ppb) | High Point (ppb) | IDL (pg) |
|---|---|---|---|---|---|---|
| N-Nitrosodimethylamine | 3.173 | 16.82 | 5 | 1000 | 2.14 | |
| Pyridine | 3.276 | 8.69 | 0.994 | 10 | 1000 | 3.23 |
| 2-Fluorophenol | 4.532 | 15.24 | 5 | 1000 | 0.28 | |
| Phenol | 5.514 | 19.25 | 1 | 1000 | 0.70 | |
| Phenol-d5 | 5.5 | 17.86 | 1 | 1000 | 0.29 | |
| Aniline | 5.554 | 9.52 | 0.993 | 10 | 1000 | 1.43 |
| Bis(2-chloroethyl) ether | 5.619 | 14.33 | 1 | 1000 | 0.42 | |
| 2-Chlorophenol | 5.688 | 19.78 | 1 | 1000 | 1.28 | |
| 1,3-Dichlorobenzene | 5.863 | 16.96 | 1 | 1000 | 0.36 | |
| 1,4-Dichlorobenzene | 5.944 | 17.90 | 1 | 1000 | 0.77 | |
| Benzyl alcohol | 6.069 | 17.06 | 1 | 1000 | 0.98 | |
| 1,2-Dichlorobenzene | 6.116 | 17.32 | 1 | 1000 | 1.13 | |
| 2-Methylphenol | 6.194 | 19.48 | 10 | 1000 | 1.95 | |
| 2,2′-oxybis(1-chloropropane) | 6.229 | 19.32 | 10 | 1000 | 4.04 | |
| 3- and 4-Methylphenol | 6.369 | 19.71 | 1 | 1000 | 1.01 | |
| N-Nitrosodi-N-propylamine | 6.374 | 5.38 | 0.997 | 10 | 1000 | 3.10 |
| Hexachloroethane | 6.511 | 15.67 | 5 | 1000 | 1.36 | |
| Nitrobenzene | 6.563 | 12.10 | 0.996 | 5 | 1000 | 0.81 |
| Nitrobenzene-d5 | 6.541 | 17.81 | 1 | 1000 | 0.96 | |
| Isophorone | 6.846 | 18.92 | 1 | 1000 | 0.56 | |
| 2-Nitrophenol | 6.941 | 13.56 | 0.996 | 10 | 1000 | 1.26 |
| 2,4-Dimethylphenol | 6.99 | 19.63 | 1 | 1000 | 0.36 | |
| Benzoic acid | 7.019 | 3.84 | 0.998 | 50 | 1000 | 14.70 |
| Bis(2-chloroethoxy)methane | 7.102 | 14.01 | 1 | 1000 | 0.26 | |
| 2,4-Dichlorophenol | 7.223 | 18.99 | 1 | 1000 | 0.83 | |
| 1,2,4-Trichlorobenzene | 7.333 | 10.67 | 1 | 1000 | 0.46 | |
| Naphthalene | 7.424 | 8.68 | 1 | 1000 | 0.73 | |
| 2,6-Dichlorophenol | 7.223 | 18.18 | 1 | 1000 | 0.62 | |
| 4-Chloroaniline | 7.484 | 13.85 | 0.998 | 5 | 1000 | 1.34 |
| Hexachlorobutadiene | 7.59 | 13.96 | 1 | 1000 | 0.65 | |
| 4-Chloro-3-methylphenol | 8.072 | 16.99 | 1 | 1000 | 0.30 | |
| 2-Methylnaphthalene | 8.268 | 10.57 | 1 | 1000 | 0.63 | |
| 1-Methylnaphthalene | 8.388 | 18.50 | 1 | 1000 | 0.64 | |
| Hexachlorocyclopentadiene | 8.474 | 16.59 | 0.998 | 5 | 1000 | 2.72 |
| 2,4,6-Trichlorophenol | 8.613 | 16.69 | 5 | 1000 | 1.55 | |
| 2,4,5-Trichlorophenol | 8.651 | 16.98 | 5 | 1000 | 0.76 | |
| 2-Fluorobiphenyl | 8.721 | 16.31 | 1 | 1000 | 1.05 | |
| 2-Chloronaphthalene | 8.862 | 14.23 | 1 | 1000 | 0.43 | |
| 2-Nitroaniline | 8.979 | 17.82 | 0.996 | 10 | 1000 | 1.33 |
| 1,4-Dinitrobenzene | 9.143 | 12.76 | 0.996 | 10 | 1000 | 8.63 |
| Dimethylphthalate | 9.216 | 18.60 | 1 | 1000 | 0.60 | |
| 1,3-Dinitrobenzene | 9.14 | 18.88 | 0.991 | 10 | 1000 | 6.97 |
| 2,6-Dinitrotoluene | 9.281 | 19.86 | 0.997 | 5 | 1000 | 2.06 |
| Acenapthylene | 9.374 | 11.73 | 1 | 1000 | 0.37 | |
| 3-Nitroaniline | 9.484 | 18.30 | 0.995 | 10 | 1000 | 2.89 |
| Acenaphthene | 9.592 | 9.44 | 0.991 | 1 | 1000 | 2.61 |
| 2,4-Dinitrophenol | 9.617 | 7.79 | 0.995 | 10 | 1000 | 11.53 |
| 4-Nitrophenol | 9.689 | 14.01 | 0.998 | 10 | 1000 | 4.39 |
| 2,4-Dinitrotoluene | 9.778 | 14.84 | 0.999 | 10 | 1000 | 4.30 |
| Dibenzofuran | 9.805 | 9.94 | 1 | 1000 | 0.38 | |
| 2,3,5,6-Tetrachlorophenol | 9.904 | 16.86 | 5 | 1000 | 2.37 | |
| 2,3,4,6-Tetrachlorophenol | 9.958 | 17.72 | 0.997 | 5 | 1000 | 1.74 |
| Diethylphthalate | 10.093 | 15.97 | 1 | 1000 | 0.67 | |
| 4-Chlorophenyl phenyl ether | 10.234 | 7.21 | 1 | 1000 | 0.57 | |
| Fluorene | 10.23 | 14.54 | 1 | 1000 | 0.37 | |
| 4-Nitroaniline | 10.234 | 18.81 | 0.993 | 10 | 1000 | 2.33 |
| 4,6-Dinitro-2-methylphenol | 10.283 | 19.99 | 0.996 | 10 | 1000 | 7.99 |
| N-Nitrosodiphenylamine | 10.376 | 18.03 | 1 | 1000 | 0.53 | |
| Diphenylamine | 10.376 | 18.03 | 1 | 1000 | 0.53 | |
| Diphenylhydrazine | 10.429 | 14.83 | 10 | 1000 | 3.39 | |
| 2,4,6-Tribromophenol | 10.524 | 18.55 | 0.997 | 5 | 1000 | 4.15 |
| 4-Bromophenyl phenyl ether | 10.839 | 15.84 | 1 | 1000 | 0.74 | |
| Hexachlorobenzene | 10.922 | 12.99 | 1 | 1000 | 0.96 | |
| Pentachlorophenol | 11.159 | 16.75 | 0.997 | 5 | 1000 | 1.14 |
| Phenanthrene | 11.421 | 13.06 | 1 | 1000 | 0.18 | |
| Anthracene | 11.484 | 15.30 | 1 | 1000 | 0.92 | |
| Carbazole | 11.68 | 14.68 | 1 | 1000 | 0.52 | |
| Di-n-butylphthalate | 12.131 | 15.80 | 5 | 1000 | 0.25 | |
| Fluoranthene | 12.9 | 10.80 | 1 | 1000 | 0.26 | |
| Benzidine | 13.062 | 19.24 | 0.998 | 10 | 1000 | 1.43 |
| Pyrene | 13.18 | 10.13 | 1 | 1000 | 0.54 | |
| o-Terphenyl-D14 | 13.377 | 13.45 | 1 | 1000 | 0.62 | |
| Butylbenzylphthalate | 14.012 | 15.67 | 5 | 1000 | 0.76 | |
| Bis(2-ethylhexyl)adipate | 14.129 | 18.95 | 0.994 | 10 | 1000 | 1.15 |
| 3’3-Dichlorobenzidine | 14.769 | 17.70 | 0.996 | 10 | 1000 | 1.76 |
| Benz[a]anthracene | 14.805 | 11.61 | 1 | 1000 | 0.38 | |
| Bis(2-ethylhexyl)phthalate | 14.875 | 19.67 | 5 | 1000 | 0.58 | |
| Chrysene | 14.861 | 8.91 | 1 | 1000 | 0.34 | |
| Di-n-octylphthalate | 16 | 18.78 | 0.998 | 5 | 1000 | 0.49 |
| Benzo[b]fluoranthene | 16.691 | 11.15 | 1 | 1000 | 0.41 | |
| Benzo[k]fluoranthene | 16.746 | 13.09 | 1 | 1000 | 0.50 | |
| Benzo[a]pyrene | 17.338 | 12.32 | 1 | 1000 | 0.24 | |
| Dibenz[a,j]acridine | 19.26 | 19.92 | 1 | 1000 | 0.60 | |
| Indeno[1,2,3-cd]pyrene | 19.754 | 15.17 | 1 | 1000 | 0.71 | |
| Dibenz[a,h]anthracene | 19.813 | 13.06 | 1 | 1000 | 0.56 | |
| Benzo[g,h,i]perylene | 20.339 | 10.79 | 1 | 1000 | 0.50 |
Conclusion
This study confirmed the suitability of RMX-5Sil MS columns for trace-level (single digit ppb) semivolatiles analysis by GC-MS/MS. Overall, the column demonstrated inertness for a broad range of compounds chemistries (acids, bases, and neutrals) and generated excellent linear calibrations. Peak shape and symmetry were intact even at low levels where active compounds are exceptionally difficult to analyze. Nearly all compounds (98%) achieved IDLs at or below single-picogram levels; calibration ranges of 1, 5, or 10 ppb to 1000 ppb; and calibration curves with ≤20% RSE.
References
- U.S. Environmental Protection Agency, Method 8270E, Semivolatile organic compounds by gas chromatography/mass spectrometry, June 2018. https://www.epa.gov/sites/default/files/2020-10/documents/method_8270e_update_vi_06-2018_0.pdf
- U.S. Environmental Protection Agency, Method 3511 (SW-846), Organic compounds in water by microextraction, July 2014. https://www.epa.gov/hw-sw846/sw-846-test-method-3511-organic-compounds-water-microextraction