If you read part three, What You’re Not Monitoring for Still Matters, of this four-part blog series, it discussed why it is important to look at additional analytes during method development to ensure there will not be any coelution with analytes that may be in your sample. In this final blog, focus will be placed on utilizing a column to its fullest potential.
It is critical that your current column is capable of resolving all analytes in your current panel. However, it is also important to know if new analytes can be added later using the same column, without sacrificing time, or consumables.
To demonstrate the ability of a column to separate analytes, varying column dimensions were used to model a set of 27 cannabinoids with one set of experimental conditions.
| Peak # | Analyte | Synonym |
| 1 | Cannabidiorcin | CBDO |
| 2 | Cannabidiethanol | CBDE |
| 3 | Cannabidivarinic acid | CBDVA |
| 4 | Cannabigerovarinic acid | CBGVA |
| 5 | Cannabidibutolic acid | CBDA |
| 6 | Cannabidibutol | CBDB |
| 7 | Cannabidiolic acid | CBDA |
| 8 | Cannabigerolic acid | CBGA |
| 9 | Cannabigerol | CBG |
| 10 | Cannabidiol | CBD |
| 11 | Tetrahydrocannabivarin | THCV |
| 12 | Cannabigerohexol | CBGH |
| 13 | Cannabichromevarin | CBCV |
| 14 | Tetrahydrocannabivarinic acid | THCVA |
| 15 | Cannabinol | CBN |
| 16 | Cannabigerophorol | CBGP |
| 17 | Cannabinolic acid | CBNA |
| 18 | Δ9-Tetrahydrocannabinol | Δ9-THC |
| 19 | Δ8-Tetrahydrocannabinol | Δ8-THC |
| 20 | (6aR,9S)-Δ10-Tetrahydrocannabinol | (6aR,9S)-Δ10-THC |
| 21 | 9(R)-Δ6a,10a Tetrahydrocannabinol | 9R-Δ6a,10a – THC |
| 22 | Cannabichromene | CBC |
| 23 | Tetrahydrocannabinolic acid A | THCA-A |
| 24 | Cannabichromenic acid | CBCA |
| 25 | Cannabicyclolic acid | CBLA |
| 26 | Cannabidiorcin | CBDO |
| 27 | Cannabidiethanol | CBDE |
| Conditions | ||
| Analytical Column: | Raptor ARC-18, 2.7 µm (varying dimensions) | |
| Flow (mL/min): | 1 | |
| Temperature (°C) | 30 | |
| % Mobile Phase A: | Water, 3 mM ammonium formate, 0.1 % formic acid | |
| % Mobile Phase B: | Acetonitrile, 0.1 % formic acid | |
| Gradient: | Isocratic 26:74 | |
| Cycle: | Time (min) | (%) B |
| 0.00 | 74 | |
| 10.00 | 74 | |
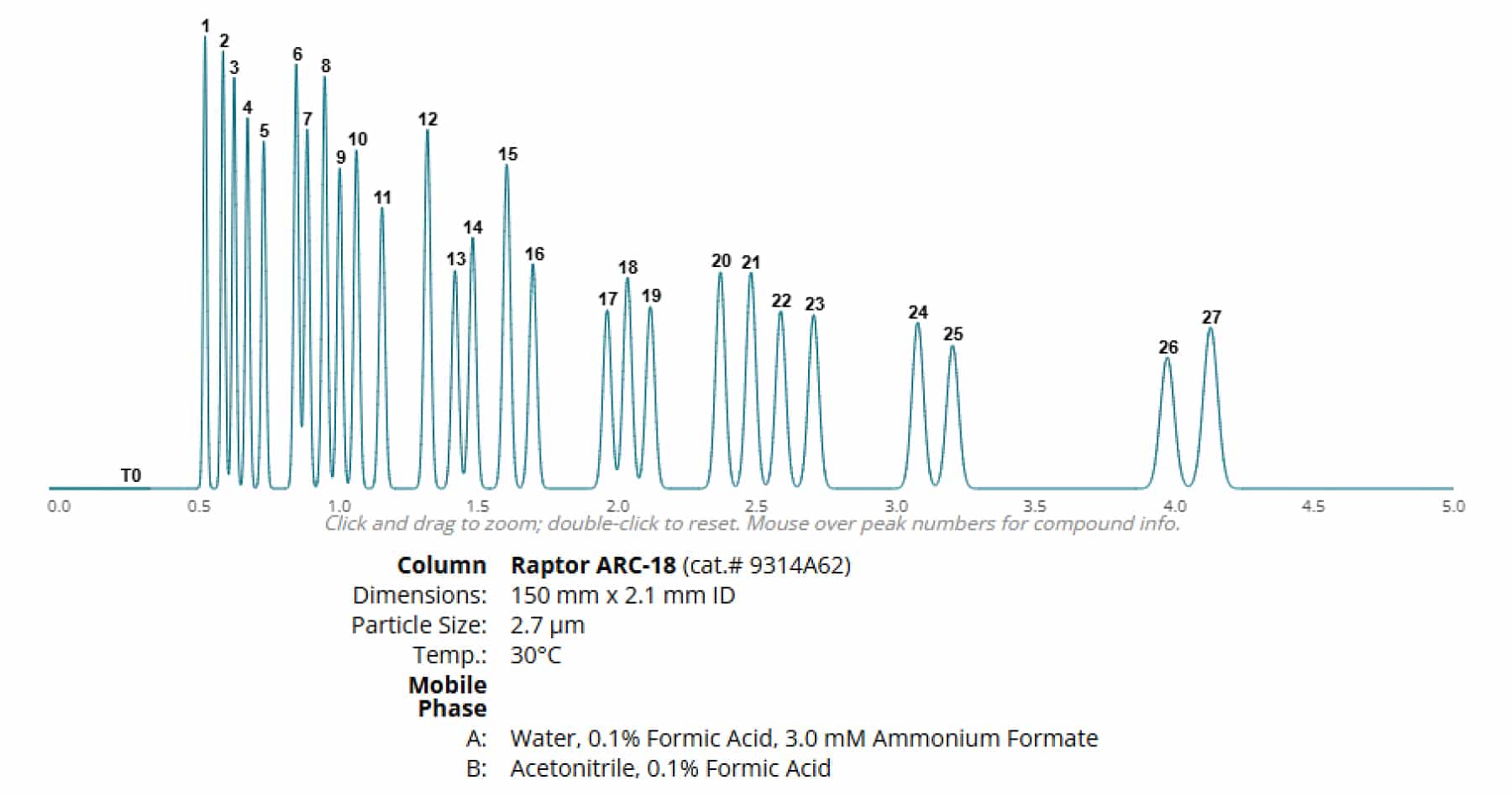
By using a 150 x 2.1 mm column, the modeler indicates that a shortened run time can be achieved, however, full resolution of analytes CBDB and CBDA cannot be achieved. Altering the method parameters causes additional coelution of analytes. Additionally, this column often has a higher pressure profile due to its dimensions along with smaller peak volumes leading to the need for a UHPLC for analysis.
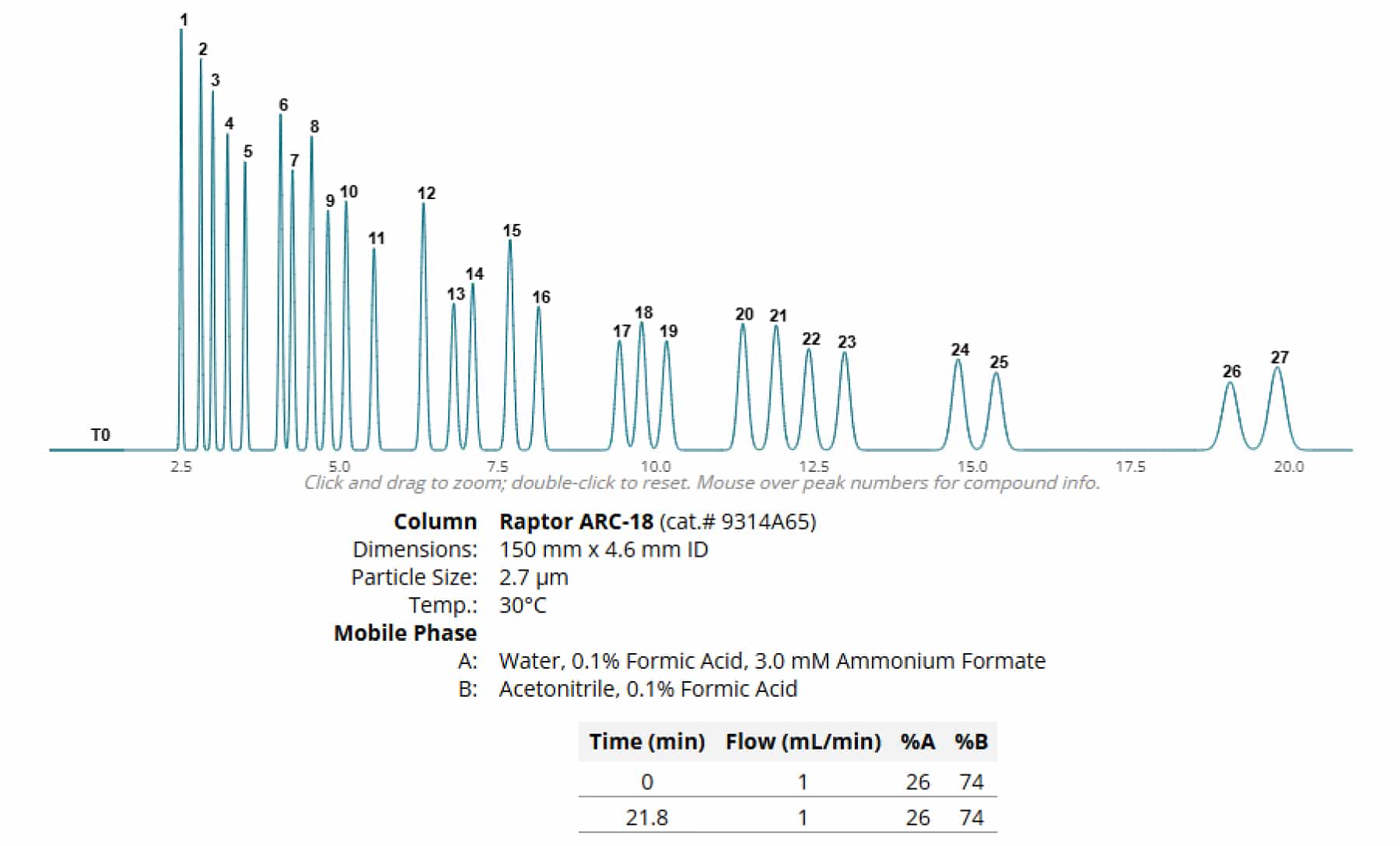
In Figure 2, using a 150 x 4.6 mm column allows for full separation of analytes, but with the caveat of a substantial increase in total run time. However, the run time can be reduced by adjusting the flow to 2.5 mL/min, giving a full cycle time of approximately nine minutes assuming the instrument can support the increase in backpressure.
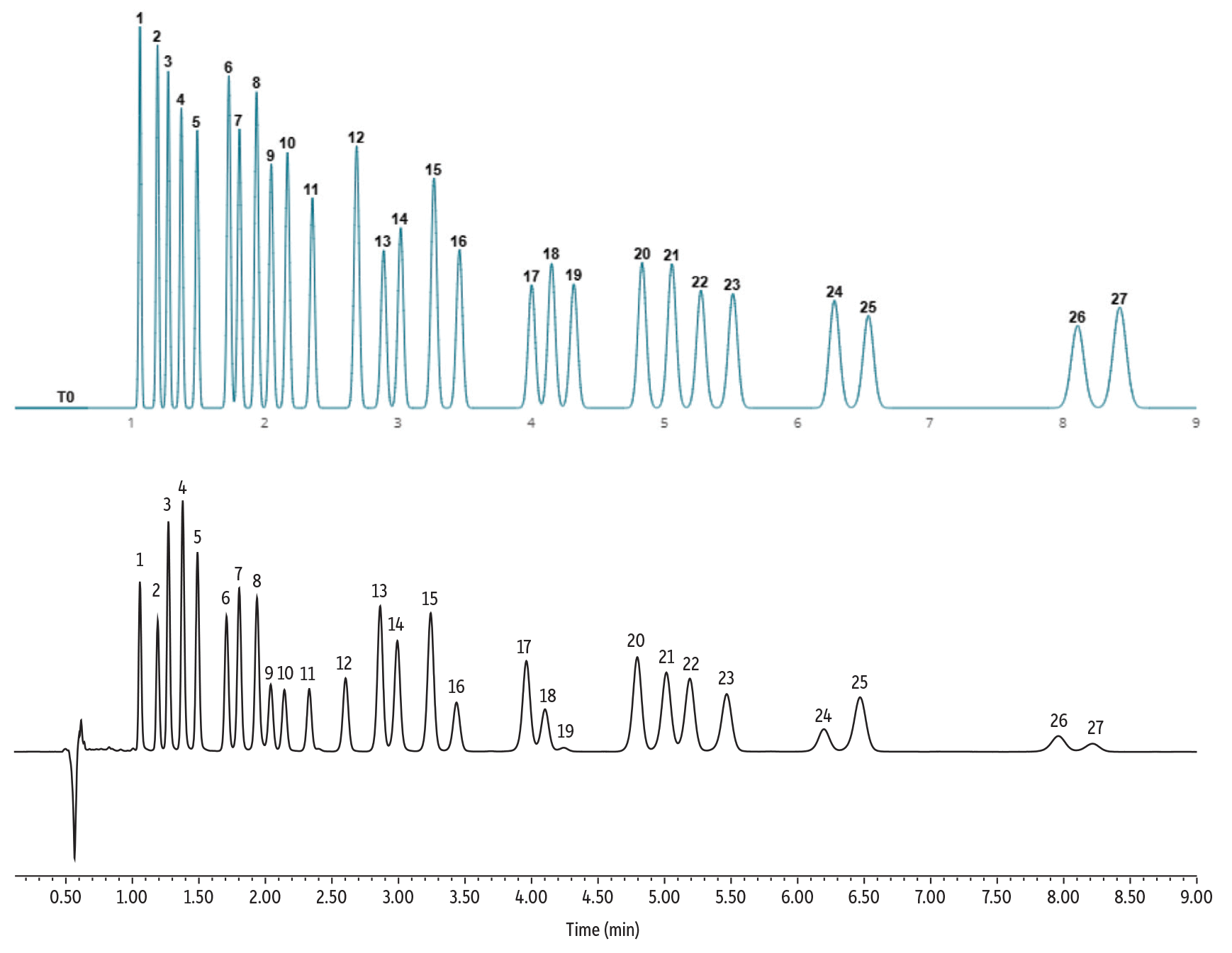
LC_GN0706
Peaks
| Peaks | Experimental tR | Modeled tR | Difference (sec) | Analytical Run Time Difference (%) | |
|---|---|---|---|---|---|
| 1. | Cannabidiorcin (CBDO) | 1.06 | 1.06 | 0.36 | 0.07 |
| 2. | Cannabidiethanol (CBDE) | 1.27 | 1.20 | 4.56 | 0.84 |
| 3. | Cannabidivarinic acid (CBDVA) | 1.38 | 1.28 | 6.12 | 1.13 |
| 4. | Cannabigerovarinic acid (CBGVA) | 1.49 | 1.37 | 6.90 | 1.28 |
| 5. | Cannabidibutolic acid (CBDBA) | 1.71 | 1.49 | 12.96 | 2.40 |
| 6. | Cannabidibutol (CBDB) | 1.80 | 1.73 | 4.32 | 0.80 |
| 7. | Cannabidiolic acid (CBDA) | 1.94 | 1.81 | 7.62 | 1.41 |
| 8. | Cannabigerolic acid (CBGA) | 2.04 | 1.94 | 6.06 | 1.12 |
| 9. | Cannabigerol (CBG) | 2.14 | 2.05 | 5.64 | 1.04 |
| 10. | Cannabidiol (CBD) | 2.33 | 2.17 | 9.48 | 1.76 |
| 11. | Tetrahydrocannabivarin (THCV) | 2.60 | 2.36 | 14.70 | 2.72 |
| 12. | Cannabigerohexol (CBGH) | 2.86 | 2.69 | 10.26 | 1.90 |
| 13. | Cannabichromevarin (CBCV) | 2.99 | 2.89 | 5.88 | 1.09 |
| Peaks | Experimental tR | Modeled tR | Difference (sec) | Analytical Run Time Difference (%) | |
|---|---|---|---|---|---|
| 14. | Tetrahydrocannabivarinic acid (THCVA) | 3.24 | 3.02 | 13.20 | 2.44 |
| 15. | Cannabinol (CBN) | 3.44 | 3.27 | 9.84 | 1.82 |
| 16. | Cannabigerophorol (CBGP) | 3.69 | 3.46 | 13.74 | 2.54 |
| 17. | Cannabinolic acid (CBNA) | 3.96 | 4.01 | 2.70 | 0.50 |
| 18. | Δ9-Tetrahydrocannabinol (Δ9-THC) | 4.10 | 4.16 | 3.24 | 0.60 |
| 19. | Δ8-Tetrahydrocannabinol (Δ8-THC) | 4.24 | 4.32 | 4.86 | 0.90 |
| 20. | (6aR,9S)-Δ10-Tetrahydrocannabinol ((6aR,9S)-∆10-THC) | 4.80 | 4.84 | 2.46 | 0.46 |
| 21. | 9(R)-∆6a,10a Tetrahydrocannabinol (9R-∆6a,10a – THC) | 5.01 | 5.06 | 2.70 | 0.50 |
| 22. | Cannabichromene (CBC) | 5.19 | 5.28 | 5.28 | 0.98 |
| 23. | Tetrahydrocannabinolic acid A (THCA-A) | 5.47 | 5.52 | 3.06 | 0.67 |
| 24. | Cannabichromenic acid (CBCA) | 6.20 | 6.28 | 5.04 | 0.93 |
| 25. | Cannabicyclolic acid (CBLA) | 6.47 | 6.54 | 4.02 | 0.74 |
| 26. | ∆9-Tetrahydrocannabiphorol (∆9-THCP) | 7.96 | 8.11 | 8.94 | 1.66 |
| 27. | Cannabicitran (CBT) | 8.22 | 8.42 | 12.48 | 2.31 |
Conditions
| Column | Raptor ARC-18 (cat.# 9314A6E) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 150 mm x 3.0 mm ID | ||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||
| Pore Size: | 90 Å | ||||||||||||
| Temp.: | 30 °C | ||||||||||||
| Standard/Sample | |||||||||||||
| Cannabinoids acids 7 standard, 1000 µg/mL, acetonitrile with 1% DIPEA and 0.05% ascorbic acid (cat.# 34144) | |||||||||||||
| Cannabinoids neutrals 9 standard, 1000 µg/mL, P&T methanol, 1 mL/ampul (cat.# 34132) | |||||||||||||
| All other cannabinoids were obtained separately. | |||||||||||||
| Diluent: | Acetonitrile | ||||||||||||
| Conc.: | 50 ppm | ||||||||||||
| Inj. Vol.: | 5 µL | ||||||||||||
| Mobile Phase | |||||||||||||
| A: | Water, 3 mM ammonium formate, 0.1 % formic acid | ||||||||||||
| B: | Acetonitrile, 0.1 % formic acid | ||||||||||||
|
| Detector | UV/Vis @ 228 nm |
|---|---|
| Flow Cell Size: | 500 nL |
| Instrument | Waters ACQUITY UPLC H-Class |
| Sample Preparation | Working standard was prepared in a 2 mL, 9 mm amber vial (cat. 21142) by diluting 50 µL of each standard into 900 µL acetonitrile and capped with a 9 mm short screw cap (cat. 24497). |
Finally, testing a 150 x 3.0 mm column dimension not only allows for full chromatographic separation, but also conserves analysis time as well as solvent consumption. By utilizing EZLC software, it is possible to determine if an existing column is capable of adding additional analytes with the current methodology, or if modifications need to be made, or a new column needs to be purchased.
Check Out the Full Blog Series in the Related Resources below.






