Abstract
A simple and reliable workflow was established here for analyzing 45 PFAS compounds in both potable and non-potable waters. The target analytes included C2 to C14 perfluoroalkyl carboxylic acids (PFCA); C1 to C13 perfluoroalkyl sulfonic acids (PFSA); fluorotelomer carboxylic acids and sulfonic acids; perfluorooctane sulfonamides and sulfonamidoacetic acids; and per- and polyfluoroether carboxylic acids and sulfonic acids. Method suitability was evaluated in terms of linearity, accuracy, precision, and suitability for a range of water types. The method produced exceptional chromatographic performance, providing a tool for comprehensive PFAS analysis that overcomes the challenges associated with conventional reversed-phase liquid chromatography (RPLC).
Introduction
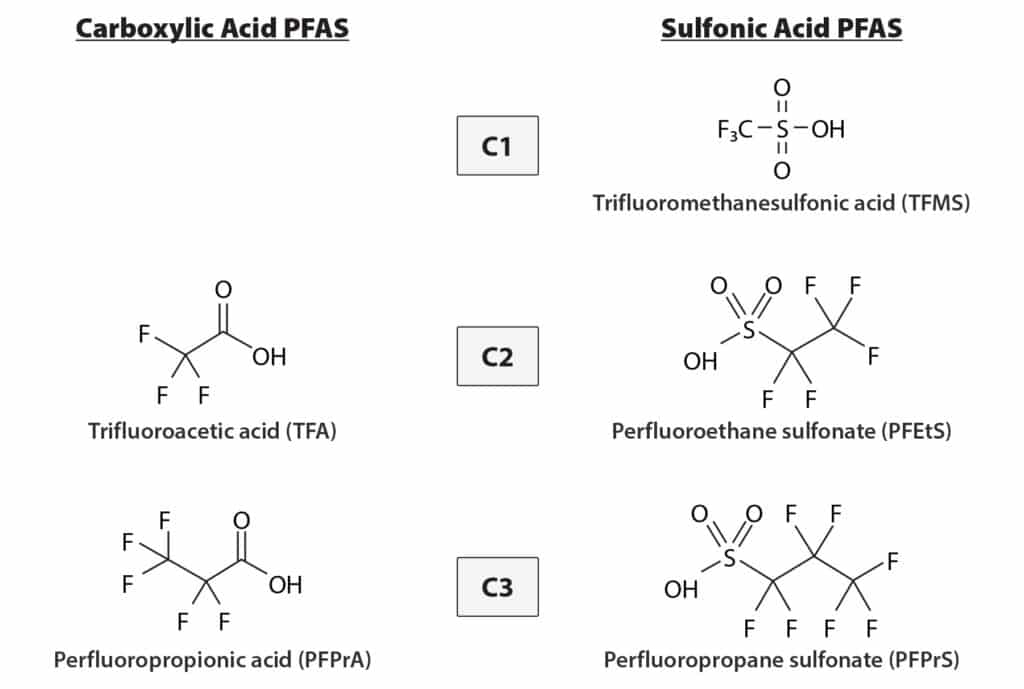
Ultrashort-chain per- and polyfluoroalkyl substances (PFAS) are small, very polar compounds with carbon chain lengths shorter than C4 (Figure 1). Their ubiquitous presence and high levels in environmental aquatic systems are emerging as a significant concern, rivaling the well-established issues associated with long-chain PFAS contamination. Therefore, it is important to analyze both ultrashort-chain and longer chain PFAS together in water samples to comprehensively assess the full spectrum of PFAS contamination. Methods allowing concurrent analysis of C1-C14 PFAS will be important tools in PFAS testing because they will allow a better understanding of environmental fate and potential human exposure, which is critical to developing effective regulations and remediation strategies.
The development of comprehensive PFAS methods that include ultrashort-chain compounds is challenging because the high polarity of ultrashort-chain PFAS makes it difficult to obtain adequate retention with an RPLC method and C18 column, which is the typical approach for analyzing C4 and longer PFAS in water. Obtaining adequate retention and separation from matrix interferences is particularly problematic for early eluting compounds, such as TFA. GC-MS has been used for analyzing TFA and C4-C6 PFCA in water samples, but it requires an extra step for PFCA derivatization and does not allow simultaneous analysis of PFSA [1]. Ultrashort-chain PFAS have also been analyzed using anion-exchange LC, but method run times exceed 20 minutes and broad peaks were observed [2]. We have previously developed a rapid method for the analysis of ultrashort- and short-chain PFAS in water samples using a hybrid HILIC and ion-exchange LC column [3], but it did not incorporate long-chain PFAS. More recently, we developed a method to quantify C1 to C10 PFAS in human plasma and serum utilizing a polar-embedded alkyl phase LC column [4]. Based on the effectiveness of that method, we used it as a starting point for the current study, which aims to develop a new method for testing a broader range of PFAS in water matrices.
The method developed here uses an LC column with a polar-embedded alkyl stationary phase to ensure adequate retention of early eluting polar compounds. In addition, the column is made with hardware treated with an inert coating, which improves sensitivity by preventing any unwanted analyte interactions with the stainless-steel column. Using this Ultra Inert IBD column, a simple and reliable workflow was developed for the simultaneous analysis of C1 to C14 perfluoroalkyl carboxylic and sulfonic acids along with other groups of PFAS. Method performance was assessed for linearity, accuracy, precision, and suitability across a wide range of potable and non-potable water matrices.
Experimental
Water Samples
Wastewater samples were gifts from General Dynamics Information Technology (Falls Church, VA). These included effluents from a publicly owned treatment works (POTW); a hospital; a metal finisher; and a chemical manufacturer. Bottled waters were obtained from local grocery stores. Tap waters were collected from Restek Corporation (Bellefonte, PA) and local households served by different borough water authorities. In addition, a natural spring water, two well waters, and three creek waters were collected from regions in central Pennsylvania.
Standard and Sample Preparation
The working calibration standard solutions (250 µL each) were prepared in reverse osmosis water across a range of 1 to 1000 ng/L in polypropylene HPLC vials. Five mass-labeled PFAS were used as quantitative internal standards (QIS) (Table I). A 2 µL aliquot of QIS working solution containing 40 ng/mL of 13C3-PFBA; 20 ng/mL of 13C2-PFHxA and 13C4-PFOA; and 10 ng/mL of 13C5-PFNA and 1313C2-PFDA was added to each standard solution, followed by mixing with 250 µL of methanol containing 1% acetic acid.
Tap water, bottled spring water, and treated sewage wastewater effluent from a POTW facility were used for the assessment of method accuracy and precision. Tap water and bottled water were used directly without filtration. The POTW water (~10 mL) was filtered with polypropylene syringe filters (cat.# 28936) and collected in 50-mL polypropylene tubes (cat.# 25846). These water samples (250 µL) were fortified at concentrations of 2, 4, 10, 50, and 250 ppt with native analytes and isotopically labeled 13C-TFA, which served as a surrogate for the determination of TFA recovery. Each fortified sample was mixed with 2 µL of QIS working solution and 2.5 µL of extracted internal standards (EIS) working solution containing 10 ng/mL of mass-labeled PFAS as detailed in Table I. A 250 µL aliquot of methanol containing 1% acetic acid was then added and mixed with the fortified samples for LC-MS/MS analysis.
Analytical System
Analysis of C1-C14 PFAS in the water samples was performed by LC-MS/MS under the conditions shown below. A PFAS delay column was installed between the mixer and injector to prevent any potential PFAS contamination upstream of the injector from coeluting with PFAS in the samples. The MS/MS transition parameters for each analyte are provided in Table I.
| System: Waters ACQUITY UPLC and Xevo TQ-S triple quadrupole mass spectrometer | |
| Columns: Analytical column: Ultra Inert IBD, 100 mm x 2.1 mm, 3 µm (cat.# 9175312-T) PFAS delay column (cat.# 27854) | |
| Injection volume: 45 µL | |
| Mobile phase A: 5 mM ammonium formate, 0.1% formic acid in water | |
| Mobile phase B: Acetonitrile | |
| Flow rate: 0.4 mL/min | |
| Temperature: 40 °C | |
| Gradient: | |
| Time (min) | %B |
| 0.00 | 50 |
| 7.00 | 95 |
| 10.00 | 95 |
| 10.01 | 50 |
| 12.00 | 50 |
| Ion mode: Negative ESI | |
| Mode: Scheduled MRM | |
Table I: MS Transitions and Analyte Retention Times
| Compounds | Time (min) | Precursor Ion | Product Ions* | Cone (V) | Collision (V) | Internal Standard |
| Target Analytes | ||||||
| Perfluoroalkyl carboxylic acids | ||||||
| Trifluoroacetic acid (TFA) | 2.12 | 113.03 [M-H]- | 69.01 | 10 | 10 | 13C3-PFBA |
| Perfluoropropanoic acid (PFPrA) | 2.69 | 162.97 [M-H]- | 119.02 | 10 | 8 | 13C3-PFBA |
| Perfluorobutanoic acid (PFBA) | 3.27 | 213.03 [M-H]- | 168.98 | 14 | 8 | 13C3-PFBA |
| Perfluoropentanoic acid (PFPeA) | 3.94 | 262.97 [M-H]- | 218.97 | 2 | 6 | 13C2-PFHxA |
| Perfluorohexanoic acid (PFHxA) | 4.59 | 313.10 [M-H]- | 268.97/118.99 | 2 | 8/20 | 13C2-PFHxA |
| Perfluoroheptanoic acid (PFHpA) | 5.24 | 363.16 [M-H]- | 319.09/169.06 | 8 | 10/18 | 13C4-PFOA |
| Perfluorooctanoic acid (PFOA) | 5.86 | 413.10 [M-H]- | 368.96/168.90 | 2 | 10/16 | 13C4-PFOA |
| Perfluorononanoic acid (PFNA) | 6.45 | 463.10 [M-H]- | 419.01/219.02 | 4 | 10/16 | 13C5-PFNA |
| Perfluorodecanoic acid (PFDA) | 7.03 | 513.17 [M-H]- | 469.16/219.06 | 4 | 12/16 | 13C2-PFDA |
| Perfluoroundecanoic acid (PFUnA) | 7.60 | 563.23 [M-H]- | 519.24/269.07 | 6 | 12/18 | 13C2-PFDA |
| Perfluorododecanoic acid (PFDoA) | 8.23 | 613.23 [M-H]- | 569.19/169.06 | 8 | 12/26 | 13C2-PFDA |
| Perfluorotridecanoic acid (PFTrDA) | 8.99 | 663.23 [M-H]- | 619.21/169.06 | 8 | 14/28 | 13C2-PFDA |
| Perfluorotetradecanoic acid (PFTeDA) | 9.83 | 712.67 [M-H]- | 668.69/168.94 | 10 | 12/26 | 13C2-PFDA |
| Perfluoroalkyl sulfonic acids | ||||||
| Trifluoromethanesulfonic acid (TFMS) | 2.36 | 148.97 [M-H]- | 79.93/98.92 | 62 | 18/18 | 13C3-PFBA |
| Perfluoroethanesulfonic acid (PFEtS) | 2.89 | 198.90 [M-H]- | 79.92/98.91 | 38 | 22/22 | 13C3-PFBA |
| Perfluoropropanesulfonic acid (PFPrS) | 3.44 | 248.97 [M-H]- | 79.92/98.91 | 2 | 24/24 | 13C3-PFBA |
| Perfluorobutanesulfonic acid (PFBS) | 3.97 | 298.97 [M-H]- | 79.97/98.89 | 2 | 26/26 | 13C2-PFHxA |
| Perfluoropentanesulfonic acid (PFPeS) | 4.50 | 349.10 [M-H]- | 79.98/98.98 | 6 | 32/30 | 13C2-PFHxA |
| Perfluorohexanesulfonic acid (PFHxS) | 5.01 | 398.90 [M-H]- | 79.97/98.89 | 56 | 32/34 | 13C2-PFHxA |
| Perfluoroheptanesulfonic acid (PFHpS) | 5.50 | 449.17 [M-H]- | 79.98/98.97 | 4 | 42/38 | 13C4-PFOA |
| Perfluorooctanesulfonic acid (PFOS) | 5.96 | 499.03 [M-H]- | 79.92/98.90 | 8 | 40/40 | 13C4-PFOA |
| Perfluorononanesulfonic acid (PFNS) | 6.38 | 549.10 [M-H]- | 79.92/98.83 | 12 | 42/40 | 13C5-PFNA |
| Perfluorodecanesulfonic acid (PFDS) | 6.77 | 599.17 [M-H]- | 79.98/98.83 | 8 | 44/46 | 13C2-PFDA |
| Perfluoroundecanesulfonic acid (PFUdS) | 7.12 | 648.73 [M-H]- | 79.94/98.94 | 38 | 50/44 | 13C2-PFDA |
| Perfluorododecanesulfonic acid (PFDoS) | 7.44 | 698.77 [M-H]- | 79.95/98.94 | 10 | 60/44 | 13C2-PFDA |
| Perfluorotridecanesulfonic acid (PFTrDS) | 7.73 | 748.73 [M-H]- | 79.94/98.94 | 8 | 76/52 | 13C2-PFDA |
| Fluorotelomer sulfonic acids | ||||||
| 1H,1H,2H,2H-Perfluorohexane sulfonic acid (4:2 FTS) | 4.22 | 327.10 [M-H]- | 307.08/80.83 | 50 | 18/24 | 13C2-PFHxA |
| 1H,1H,2H,2H-Perfluorooctane sulfonic acid (6:2 FTS) | 5.75 | 427.17 [M-H]- | 407.18/80.71 | 2 | 22/32 | 13C4-PFOA |
| 1H,1H,2H,2H-Perfluorodecane sulfonic acid (8:2 FTS) | 7.22 | 527.17 [M-H]- | 507.16/80.83 | 66 | 26/32 | 13C2-PFDA |
| Fluorotelomer carboxylic acids | ||||||
| 3-Perfluoropropyl propanoic acid (3:3 FTCA) | 1.64 | 241.00 [M-H]- | 177.00/117.00 | 2 | 6/6 | 13C3-PFBA |
| 3-Perfluoropentyl propanoic acid (5:3 FTCA) | 2.49 | 340.93 [M-H]- | 216.96/236.93 | 2 | 24/14 | 13C3-PFBA |
| 3-Perfluoroheptyl propanoic acid (7:3 FTCA) | 3.61 | 440.90 [M-H]- | 336.88/316.91 | 20 | 12/22 | 13C3-PFBA |
| Perfluorooctane sulfonamides | ||||||
| Perfluorooctanesulfonamide (FOSA) | 3.36 | 498.17 [M-H]- | 77.97/477.76 | 8 | 28/26 | 13C3-PFBA |
| N-methyl perfluorooctanesulfonamide (NMeFOSA) | 3.96 | 511.77 [M-H]- | 168.95/218.91 | 2 | 26/24 | 13C2-PFHxA |
| N-ethyl perfluorooctanesulfonamide (NEtFOSA) | 4.26 | 525.83 [M-H]- | 168.96/218.92 | 10 | 26/24 | 13C2-PFHxA |
| Perfluorooctane sulfonamidoacetic acids | ||||||
| N-methyl perfluorooctanesulfonamidoacetic acid (NMeFOSAA) | 6.44 | 570.20 [M-H]- | 419.17/483.16 | 46 | 20/14 | 13C5-PFNA |
| N-ethyl perfluorooctanesulfonamidoacetic acid (NEtFOSAA) | 6.56 | 584.20 [M-H]- | 419.18/483.11 | 6 | 20/16 | 13C5-PFNA |
| Per- and polyfluoroether carboxylic acids | ||||||
| Perfluoro-3-methoxypropanoic acid (PFMPA) | 3.40 | 228.93 [M-H]- | 84.97/198.94 | 10 | 10/14 | 13C3-PFBA |
| Perfluoro-4-methoxybutanoic acid (PFMBA) | 4.00 | 278.87 [M-H]- | 84.96/234.93 | 8 | 10/6 | 13C2-PFHxA |
| Nonafluoro-3,6-dioxaheptanoic acid (NFDHA) | 4.06 | 294.93 [M-H]- | 200.91/85.02 | 8 | 4/22 | 13C2-PFHxA |
| Hexafluoropropylene oxide dimer acid (HFPO-DA) | 4.46 | 285.03 [M-COOH]- | 169.02/185.02 | 2 | 6/16 | 13C2-PFHxA |
| 4,8-Dioxa-3H-perfluorononanoic acid (ADONA) | 4.63 | 376.90 [M-H]- | 250.93/84.97 | 22 | 12/26 | 13C2-PFHxA |
| Ether sulfonic acids | ||||||
| Perfluoro(2-ethoxyethane)sulfonic acid (PFEESA) | 4.04 | 314.83 [M-H]- | 134.94/83.01 | 4 | 22/16 | 13C2-PFHxA |
| 9-Chlorohexadecafluoro-3-oxanonane-1-sulfonic acid (9Cl-PF3ONS) | 5.88 | 530.78 [M-H]- | 350.85/82.96 | 12 | 26/24 | 13C4-PFOA |
| 11-Chloroeicosafluoro-3-oxaundecane-1-sulfonic acid (11Cl-PF3OUdS) | 6.56 | 630.78 [M-H]- | 450.80/82.95 | 8 | 26/32 | 13C5-PFNA |
| Extracted Internal Standards | ||||||
| 13C3-PFPrA | 2.69 | 165.97 [M-H]- | 120.96 | 10 | 11 | 13C3-PFBA |
| 13C4-PFBA | 3.27 | 217.03 [M-H]- | 171.98 | 2 | 8 | 13C3-PFBA |
| 13C5-PFPeA | 3.94 | 267.97 [M-H]- | 222.99 | 2 | 6 | 13C2-PFHxA |
| 13C5-PFHxA | 4.59 | 318.03 [M-H]- | 272.93 | 2 | 7 | 13C2-PFHxA |
| 13C4-PFHpA | 5.24 | 366.90 [M-H]- | 321.93 | 2 | 10 | 13C4-PFOA |
| 13C8-PFOA | 5.86 | 420.97 [M-H]- | 375.94 | 2 | 10 | 13C4-PFOA |
| 13C6-PFDA | 7.03 | 518.90 [M-H]- | 473.87 | 4 | 13 | 13C2-PFDA |
| 13C7-PFUnA | 7.60 | 569.90 [M-H]- | 524.87 | 2 | 12 | 13C2-PFDA |
| 13C2-PFDoA | 8.23 | 614.84 [M-H]- | 569.87 | 2 | 12 | 13C2-PFDA |
| 13C2-PFTeDA | 9.83 | 714.78 [M-H]- | 669.80 | 8 | 14 | 13C2-PFDA |
| 13C3-PFBS | 3.97 | 301.97 [M-H]- | 79.97 | 2 | 28 | 13C2-PFHxA |
| 13C3-PFHxS | 5.01 | 401.90 [M-H]- | 79.97 | 2 | 36 | 13C2-PFHxA |
| 13C8-PFOS | 5.96 | 506.84 [M-H]- | 79.97 | 4 | 42 | 13C4-PFOA |
| 13C2-4:2 FTS | 4.22 | 328.97 [M-H]- | 308.96 | 2 | 18 | 13C2-PFHxA |
| 13C2-8:2 FTS | 7.22 | 528.90 [M-H]- | 508.90 | 2 | 24 | 13C2-PFDA |
| 13C8-FOSA | 3.36 | 505.91 [M-H]- | 77.95 | 4 | 32 | 13C3-PFBA |
| d3-NMeFOSAA | 6.44 | 572.90 [M-H]- | 418.91 | 50 | 18 | 13C5-PFNA |
| d5-NEtFOSAA | 6.56 | 588.97 [M-H]- | 418.86 | 48 | 20 | 13C5-PFNA |
| Quantification Internal Standards | ||||||
| 13C3-PFBA | 3.27 | 215.97 [M-H]- | 171.97 | 10 | 8 | – |
| 13C2-PFHxA | 4.59 | 314.97 [M-H]- | 269.93 | 8 | 8 | – |
| 13C4-PFOA | 5.86 | 416.87 [M-H]- | 371.88 | 2 | 8 | – |
| 13C5-PFNA | 6.45 | 467.87 [M-H]- | 422.89 | 16 | 10 | – |
| 13C2-PFDA | 7.03 | 514.87 [M-H]- | 469.84 | 8 | 10 | – |
*Quantifier ion/qualifier ion.
Results and Discussion
LC-MS/MS Method Development
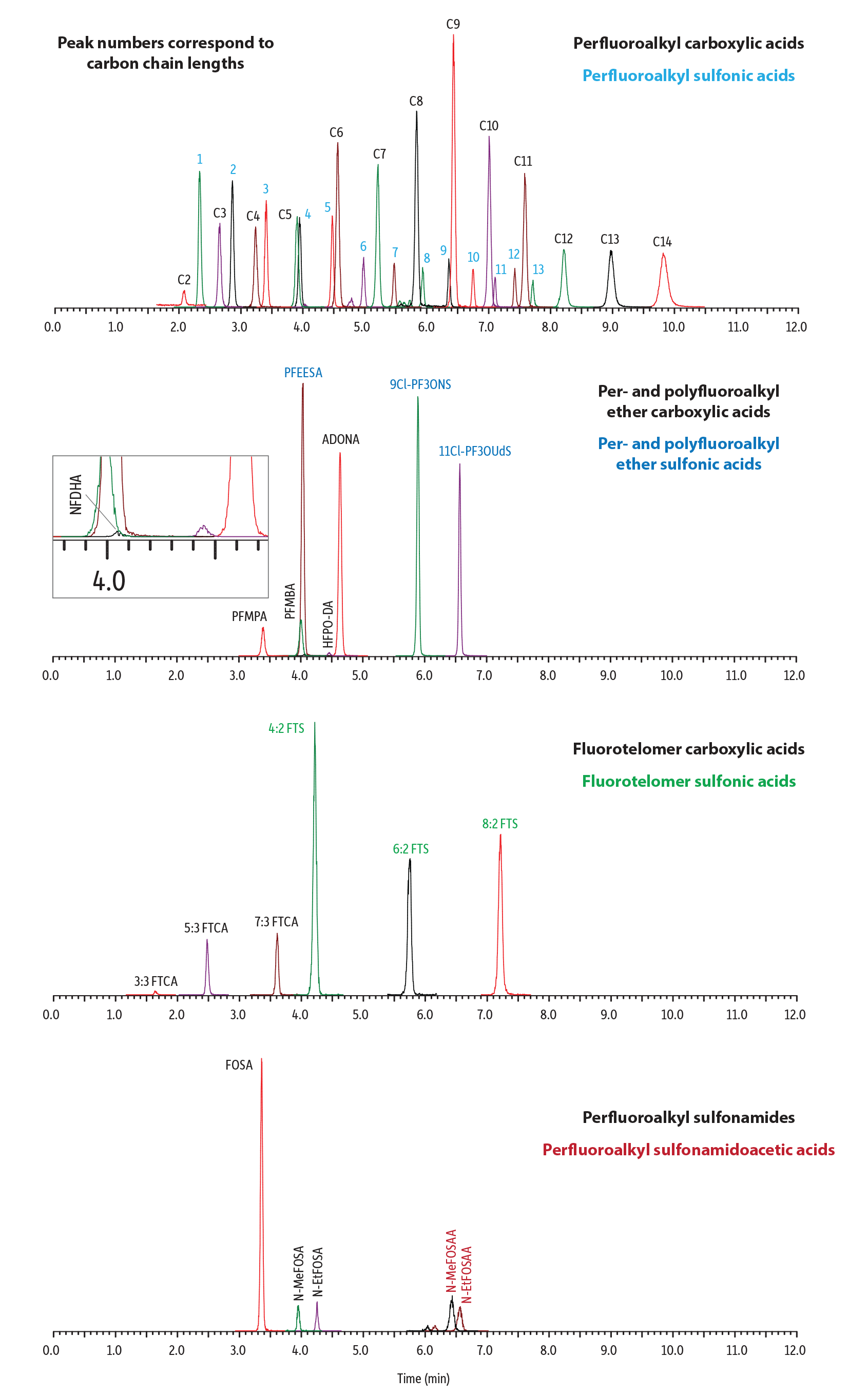
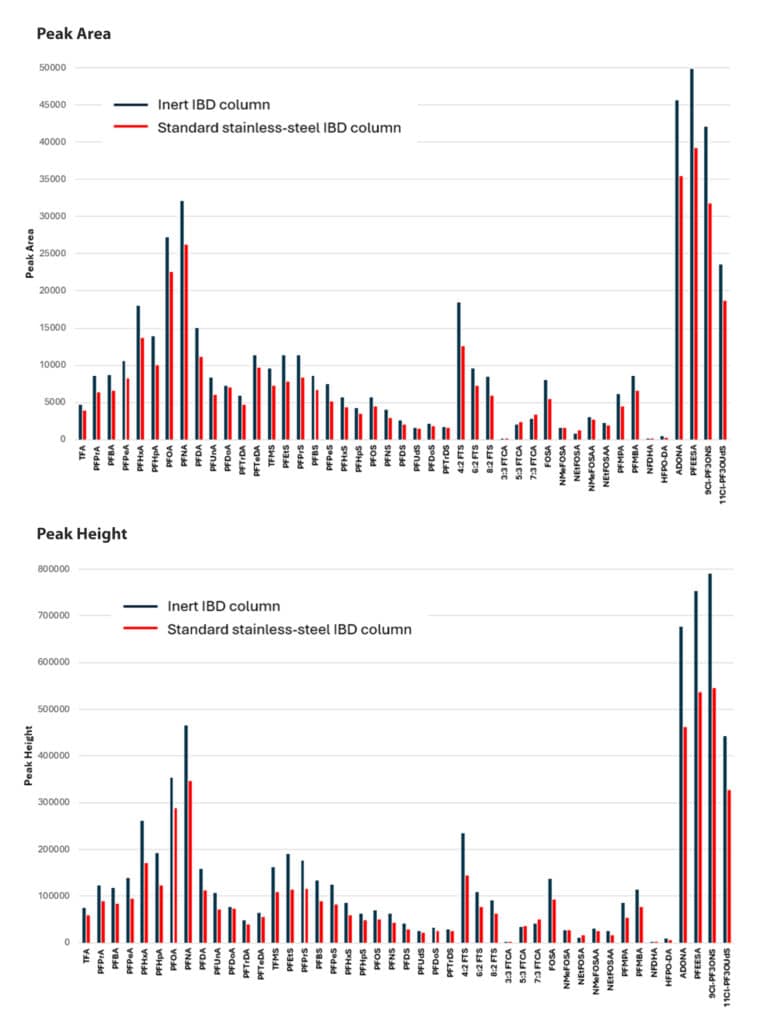
An effective chromatographic method was established for the comprehensive analysis of 45 PFAS, including ultrashort-chain compounds, in potable and non-potable water samples (Figure 2). The Ultra Inert IBD column exhibited excellent chromatographic performance under reversed-phased conditions, with the embedded polar group providing good retention of TFA and other early eluting PFAS. In addition, as shown in Figure 3, the inert hardware resulted in a notable enhancement in detection sensitivity for most of the analytes (3-70% increase in peak area and 5-75% increase in peak height) compared to columns with uncoated hardware.
Linearity
Employing quadratic regression (1/x weighted), all analytes exhibited acceptable linearities with r2 >0.995 and deviations <30%. Table II shows the different linearity ranges for each target PFAS, which ranged from 1 ppt to 1000 ppt, with variation occurring at the lowest calibration concentration. LOQ and LOD values are also presented (LOQ values were defined as the concentration of the lowest calibration standard for each analyte).
Table II: LOQ* and LOD Values for PFAS in Tap, Bottled, and POTW Water
| Linearity Range | LOD (ng/L) | |||
| Analytes | (ng/L) | Tap Water | Bottled Water | POTW |
| TFA | 10–1000 | 1.7 | 1.4 | 2.1 |
| PFPrA | 1–1000 | 0.3 | 0.3 | 0.3 |
| PFBA | 2–1000 | 0.7 | 0.6 | 0.6 |
| PFPeA | 1–1000 | 0.5 | 0.4 | 0.3 |
| PFHxA | 1–1000 | 0.4 | 0.4 | 0.4 |
| PFHpA | 1–1000 | 0.4 | 0.4 | 0.5 |
| PFOA | 1–1000 | 0.4 | 0.4 | 0.4 |
| PFNA | 1–1000 | 0.3 | 0.3 | 0.3 |
| PFDA | 1–1000 | 0.4 | 0.3 | 0.5 |
| PFUnA | 1–1000 | 0.5 | 0.3 | 0.4 |
| PFDoA | 2–1000 | 0.7 | 0.4 | 0.5 |
| PFTrDA | 2–1000 | 0.8 | 0.6 | 0.6 |
| PFTeDA | 2–1000 | 0.8 | 0.7 | 0.6 |
| TFMS | 1–1000 | 0.2 | 0.2 | 0.2 |
| PFEtS | 1–1000 | 0.1 | 0.3 | 0.2 |
| PFPrS | 1–1000 | 0.2 | 0.2 | 0.4 |
| PFBS | 1–1000 | 0.2 | 0.2 | 0.5 |
| PFPeS | 1–1000 | 0.2 | 0.3 | 0.2 |
| PFHxS | 1–1000 | 0.3 | 0.6 | 0.5 |
| PFHpS | 2–1000 | 0.5 | 0.4 | 0.4 |
| PFOS | 2–1000 | 0.5 | 0.9 | 0.8 |
| PFNS | 2–1000 | 0.6 | 0.6 | 0.6 |
| PFDS | 2–1000 | 0.6 | 0.7 | 0.9 |
| PFUdS | 2–1000 | 0.5 | 0.7 | 0.9 |
| PFDoS | 2–1000 | 0.4 | 0.6 | 0.6 |
| PFTrDS | 2–1000 | 0.9 | 0.6 | 0.8 |
| 4:2 FTS | 2–1000 | 0.4 | 0.6 | 0.5 |
| 6:2 FTS | 2–1000 | 0.6 | 0.4 | 0.5 |
| 8:2 FTS | 2–1000 | 0.4 | 0.4 | 0.5 |
| 3:3 FTCA | 20–1000 | 8.3 | 7.4 | – |
| 5:3 FTCA | 4–1000 | 1.2 | 1.1 | 1.5 |
| 7:3 FTCA | 4–1000 | 1.6 | 1.3 | 1.2 |
| FOSA | 1–1000 | 0.2 | 0.2 | 0.2 |
| NMeFOSA | 10–1000 | 3.8 | 4.0 | 4.0 |
| NEtFOSA | 10–1000 | 4.6 | 4.0 | 4.0 |
| NMeFOSAA | 4–1000 | 1.2 | 1.2 | 1.9 |
| NEtFOSAA | 10–1000 | 4.3 | 2.5 | 3.3 |
| PFMPA | 1–1000 | 0.3 | 0.2 | 0.4 |
| PFMBA | 1–1000 | 0.4 | 0.3 | 0.4 |
| NFDHA | 20–1000 | 10.0 | 8.8 | 7.9 |
| HFPO-DA | 10–1000 | 3.8 | 3.3 | 5.2 |
| ADONA | 1–1000 | 0.1 | 0.1 | 0.2 |
| PFEESA | 1–1000 | 0.1 | 0.1 | 0.1 |
| 9Cl-PF3ONS | 1–1000 | 0.2 | 0.2 | 0.2 |
| 11Cl-PF3OUdS | 1–1000 | 0.2 | 0.1 | 0.3 |
*LOQ = concentration of the lowest calibration standard solution.
Accuracy and Precision
Three batches of samples were analyzed on different days, totaling nine replicates for each fortification level. The average recoveries and relative standard deviations (RSD) are presented in Tables III-V. All analytes exhibited good recovery values within the range of 70–130% across all fortification levels. Satisfactory method precision was demonstrated by %RSD values of <20%. Additionally, the results indicated that all extracted internal standards (EIS) had recovery values within 30% of the nominal concentration.
Table III: Accuracy and Precision Results for Tap Water Samples
| Analytes | Average Recovery (RSD, %), n=9 | ||||
| Fortified Concentration (ng/L) | |||||
| 2 | 4 | 10 | 50 | 250 | |
| 13C-TFA | – | – | 110 (9.61) | 89.8 (5.10) | 91.5 (2.04) |
| PFPrA | 102 (6.88) | 100 (3.72) | 98.6 (6.71) | 105 (8.98) | 101 (6.85) |
| PFBA | 108 (8.17) | 107 (7.28) | 104 (9.06) | 104 (5.13) | 103 (7.20) |
| PFPeA | 109 (9.02) | 115 (2.98) | 105 (7.90) | 111 (6.13) | 107 (8.17) |
| PFHxA | 88.2 (8.36) | 99.8 (8.44) | 92.0 (6.26) | 105 (7.96) | 103 (8.06) |
| PFHpA | 88.7 (6.44) | 101 (8.38) | 86.2 (6.26) | 95.1 (6.97) | 91.2 (7.28) |
| PFOA | 111 (6.58) | 117 (4.67) | 103 (9.51) | 104 (8.34) | 101 (8.98) |
| PFNA | 108 (7.53) | 110 (5.46) | 97.8 (3.83) | 104 (6.23) | 99.5 (8.40) |
| PFDA | 99.4 (6.34) | 104 (5.56) | 95.1 (5.35) | 98.6 (9.37) | 95.8 (8.66) |
| PFUnA | 110 (14.0) | 109 (11.4) | 108 (14.6) | 107 (8.05) | 95.5 (7.14) |
| PFDoA | 103 (14.2) | 104 (9.98) | 96.5 (14.1) | 101 (5.81) | 102 (3.95) |
| PFTrDA | 101 (13.3) | 91.1 (11.0) | 87.5 (7.09) | 82.3 (5.23) | 86.5 (3.05) |
| PFTeDA | 103 (6.04) | 91.2 (10.6) | 90.1 (8.18) | 86.5 (10.9) | 93.4 (4.21) |
| TFMS | 112 (9.16) | 108 (10.4) | 97.3 (8.16) | 105 (6.42) | 104 (7.25) |
| PFEtS | 103 (8.31) | 115 (5.52) | 103 (3.25) | 106 (4.64) | 107 (5.60) |
| PFPrS | 109 (9.22) | 114 (7.23) | 99.3 (8.52) | 106 (7.76) | 107 (7.32) |
| PFBS | 109 (6.89) | 109 (10.7) | 94.3 (8.59) | 101 (5.45) | 102 (6.53) |
| PFPeS | 104 (5.33) | 113 (3.79) | 97.9 (4.84) | 106 (7.29) | 104 (9.30) |
| PFHxS | 84.1 (16.7) | 105 (7.50) | 90.8 (12.8) | 104 (4.37) | 102 (3.77) |
| PFHpS | 111 (7.72) | 108 (6.82) | 99.7 (7.04) | 108 (5.65) | 105 (6.59) |
| PFOS | 112 (7.55) | 106 (7.10) | 95.5 (10.2) | 107 (7.39) | 107 (6.83) |
| PFNS | 117 (13.8) | 104 (10.7) | 91.9 (10.2) | 104 (4.92) | 99.1 (6.15) |
| PFDS | 107 (15.0) | 106 (8.32) | 94.4 (17.2) | 97.9 (11.1) | 94.4 (3.40) |
| PFUdS | 108 (13.5) | 92.4 (15.5) | 88.1 (1.58) | 85.7 (2.32) | 93.4 (3.38) |
| PFDoS | 114 (13.4) | 96.5 (11.9) | 85.9 (5.01) | 83.5 (11.6) | 93.9 (2.71) |
| PFTrDS | 91.0 (17.8) | 72.5 (18.2) | 72.0 (13.8) | 72.9 (14.4) | 84.6 (3.55) |
| 4:2 FTS | 106 (8.72) | 109 (8.83) | 90.2 (13.0) | 103 (9.27) | 101 (10.2) |
| 6:2 FTS | 108 (8.94) | 112 (6.81) | 96.4 (17.3) | 102 (4.45) | 99.4 (7.60) |
| 8:2 FTS | 111 (13.4) | 107 (12.1) | 92.6 (11.3) | 101 (5.28) | 94.6 (5.86) |
| 3:3 FTCA | – | – | – | 83.7 (8.92) | 82.6 (5.64) |
| 5:3 FTCA | – | 120 (6.07) | 106 (12.0) | 103 (8.99) | 103 (8.78) |
| 7:3 FTCA | – | 107 (8.36) | 91.4 (16.2) | 105 (7.98) | 106 (7.36) |
| FOSA | 107 (9.41) | 107 (5.20) | 95.4 (8.59) | 95.7 (4.63) | 95.5 (6.05) |
| NMeFOSA | – | – | 106 (12.9) | 105 (6.50) | 104 (9.06) |
| NEtFOSA | – | – | 105 (14.7) | 112 (4.89) | 105 (7.49) |
| NMeFOSAA | – | 123 (7.06) | 88.5 (14.6) | 102 (8.37) | 99.5 (7.89) |
| NEtFOSAA | – | – | 106 (10.9) | 96.1 (11.1) | 92.6 (5.99) |
| PFMPA | 99.0 (15.9) | 103 (10.6) | 90.2 (6.11) | 103 (8.22) | 103 (8.21) |
| PFMBA | 105 (7.67) | 107 (8.82) | 95.8 (7.63) | 107 (6.27) | 103 (7.01) |
| NFDHA | – | – | – | 115 (9.78) | 115 (8.61) |
| HFPO-DA | – | – | 115 (13.1) | 108 (5.70) | 106 (7.76) |
| ADONA | 94.4 (11.5) | 109 (5.71) | 98.4 (7.58) | 106 (7.56) | 104 (8.94) |
| PFEESA | 102 (8.88) | 113 (4.75) | 98.8 (7.11) | 108 (6.06) | 105 (8.76) |
| 9Cl-PF3ONS | 100 (8.99) | 107 (4.54) | 95.7 (2.88) | 105 (6.31) | 104 (9.09) |
| 11Cl-PF3OUdS | 88.2 (12.3) | 96.8 (7.36) | 87.8 (8.81) | 96.7 (4.65) | 95.1 (4.17) |
Table IV: Accuracy and Precision Results for Bottled Water Samples
| Analytes | Average Recovery (RSD, %), n=9 | ||||
| Fortified Concentration (ng/L) | |||||
| 2 | 4 | 10 | 50 | 250 | |
| 13C-TFA | – | – | 104 (15.6) | 99.4 (8.09) | 103 (7.45) |
| PFPrA | 96.7 (5.90) | 101 (6.64) | 97.9 (6.08) | 107 (3.13) | 114 (7.77) |
| PFBA | 96.4 (5.81) | 94.7 (8.49) | 90.2 (6.21) | 103 (6.58) | 112 (6.78) |
| PFPeA | 93.2 (8.17) | 99.1 (13.9) | 95.5 (10.2) | 105 (5.95) | 111 (8.53) |
| PFHxA | 100 (13.1) | 96.5 (9.30) | 91.0 (8.66) | 99.5 (4.92) | 108 (8.20) |
| PFHpA | 96.9 (8.26) | 92.3 (3.67) | 87.3 (4.82) | 97.9 (6.15) | 104 (5.56) |
| PFOA | 94.4 (10.9) | 100 (10.8) | 101 (8.29) | 105 (6.09) | 112 (6.11) |
| PFNA | 108 (9.34) | 103 (8.32) | 97.3 (3.99) | 105 (5.46) | 112 (7.09) |
| PFDA | 101 (11.9) | 96.9 (6.73) | 92.2 (8.25) | 100 (5.31) | 109 (8.86) |
| PFUnA | 109 (10.6) | 104 (8.74) | 90.6 (11.0) | 97.7 (9.52) | 105 (5.72) |
| PFDoA | 97.5 (5.77) | 95.2 (4.93) | 78.5 (1.90) | 85.1 (5.14) | 103 (5.68) |
| PFTrDA | 106 (9.03) | 100 (8.97) | 81.8 (13.1) | 87.8 (18.6) | 98.2 (9.05) |
| PFTeDA | 116 (3.97) | 109 (6.74) | 90.5 (8.94) | 93.5 (18.2) | 114 (5.74) |
| TFMS | 101 (9.04) | 107 (6.85) | 96.7 (4.30) | 106 (2.88) | 112 (7.17) |
| PFEtS | 102 (7.45) | 105 (4.66) | 97.8 (3.00) | 106 (2.01) | 111 (7.50) |
| PFPrS | 101 (10.4) | 106 (7.28) | 98.1 (6.13) | 108 (1.98) | 111 (6.44) |
| PFBS | 103 (13.7) | 101 (9.53) | 85.8 (5.98) | 101 (2.59) | 108 (8.90) |
| PFPeS | 95.6 (10.3) | 102 (5.75) | 92.3 (5.80) | 102 (2.79) | 109 (9.05) |
| PFHxS | 110 (8.75) | 99.4 (8.55) | 90.9 (11.8) | 96.5 (2.79) | 103 (8.67) |
| PFHpS | 99.8 (8.79) | 94.2 (8.80) | 90.9 (7.75) | 105 (5.47) | 110 (8.06) |
| PFOS | 111 (12.9) | 93.5 (8.65) | 91.8 (9.70) | 106 (6.02) | 109 (6.08) |
| PFNS | 102 (12.8) | 90.8 (15.2) | 86.8 (10.4) | 101 (8.80) | 112 (6.74) |
| PFDS | 96.9 (19.1) | 105 (12.1) | 89.3 (11.4) | 103 (10.2) | 107 (5.82) |
| PFUdS | 101 (18.5) | 100 (16.3) | 87.3 (10.5) | 92.9 (14.5) | 105 (5.71) |
| PFDoS | 114 (7.52) | 106 (10.7) | 84.8 (14.6) | 90.7 (14.5) | 108 (7.49) |
| PFTrDS | 126 (1.13) | 109 (14.4) | 86.2 (17.1) | 88.4 (19.0) | 112 (5.51) |
| 4:2 FTS | 103 (11.0) | 98.1 (16.3) | 88.6 (10.1) | 94.3 (4.60) | 108 (10.3) |
| 6:2 FTS | 108 (6.44) | 95.2 (13.7) | 88.0 (13.3) | 97.7 (9.07) | 106 (7.53) |
| 8:2 FTS | 112 (10.5) | 109 (5.14) | 95.0 (3.66) | 103 (3.89) | 109 (8.72) |
| 3:3 FTCA | – | – | – | 95.4 (10.4) | 105 (6.94) |
| 5:3 FTCA | – | 103 (8.78) | 94.3 (12.1) | 105 (6.32) | 109 (8.62) |
| 7:3 FTCA | – | 115 (5.16) | 98.9 (9.92) | 102 (8.75) | 110 (7.09) |
| FOSA | 111 (7.75) | 103 (8.73) | 91.6 (3.72) | 104 (3.88) | 112 (7.06) |
| NMeFOSA | – | – | 105 (8.01) | 101 (10.8) | 115 (5.88) |
| NEtFOSA | – | – | 121 (9.43) | 100 (13.0) | 110 (8.06) |
| NMeFOSAA | – | 109 (12.1) | 106 (10.1) | 92.9 (13.8) | 104 (9.53) |
| NEtFOSAA | – | – | 101 (15.7) | 100 (10.3) | 105 (8.11) |
| PFMPA | 99.5 (9.11) | 99.6 (11.0) | 92.2 (4.76) | 103 (4.57) | 112 (7.29) |
| PFMBA | 92.3 (9.01) | 102 (9.17) | 90.1 (8.50) | 100 (4.51) | 109 (7.70) |
| NFDHA | – | – | – | 111 (6.59) | 111 (7.73) |
| HFPO-DA | – | – | 106 (12.3) | 101 (10.1) | 112 (6.19) |
| ADONA | 97.3 (11.4) | 101 (6.41) | 91.6 (3.35) | 100 (4.26) | 107 (10.3) |
| PFEESA | 101 (8.99) | 105 (8.33) | 93.6 (5.67) | 102 (3.03) | 110 (9.16) |
| 9Cl-PF3ONS | 100 (8.53) | 101 (7.16) | 90.2 (4.28) | 105 (4.22) | 112 (8.47) |
| 11Cl-PF3OUdS | 91.0 (11.1) | 96.8 (6.21) | 85.0 (4.94) | 100 (11.8) | 108 (5.75) |
Table V: Accuracy and Precision Results for POTW Water Samples
| Analytes | Average Recovery (RSD, %), n=9 | ||||
| Fortified Concentration (ng/L) | |||||
| 2 | 4 | 10 | 50 | 250 | |
| 13C-TFA | – | – | – | 104 (9.46) | 108 (8.57) |
| PFPrA | 99.7 (3.96) | 101 (11.9) | 99.7 (9.18) | 109 (3.90) | 113 (1.99) |
| PFBA | 100 (8.79) | 98.0 (6.35) | 99.2 (7.44) | 104 (8.77) | 106 (4.82) |
| PFPeA | 104 (9.17) | 110 (6.19) | 101 (5.66) | 109 (2.80) | 116 (2.05) |
| PFHxA | 91.8 (12.1) | 98.5 (10.0) | 92.9 (9.53) | 101(3.30) | 103 (6.53) |
| PFHpA | 115 (5.90) | 111 (8.16) | 97.6 (6.90) | 97.1 (5.74) | 99.5 (4.58) |
| PFOA | 108 (8.67) | 105 (8.15) | 101 (4.86) | 105 (4.68) | 109 (5.20) |
| PFNA | 113 (8.50) | 117 (5.50) | 95.1 (5.73) | 103 (6.97) | 107 (4.67) |
| PFDA | 108 (10.6) | 111 (8.44) | 91.8 (5.90) | 97.6 (4.10) | 101 (3.49) |
| PFUnA | 106 (12.6) | 102 (9.97) | 95.1 (8.99) | 97.7 (3.90) | 101 (6.28) |
| PFDoA | 98.4 (16.6) | 107 (9.05) | 93.0 (3.83) | 111 (11.9) | 112 (12.2) |
| PFTrDA | 94.1 (15.9) | 94.4 (13.2) | 79.7 (9.70) | 90.3 (11.3) | 96.7 (13.5) |
| PFTeDA | 113 (12.7) | 97.5 (18.9) | 82.9 (12.4) | 95.2 (13.3) | 104 (18.0) |
| TFMS | 104 (15.6) | 108 (7.24) | 94.9 (5.45) | 94.9 (5.32) | 96.0 (1.88) |
| PFEtS | 93.1 (15.3) | 108 (7.71) | 87.9 (6.50) | 95.7 (4.51) | 97.9 (2.89) |
| PFPrS | 105 (9.36) | 114 (4.26) | 91.5 (4.65) | 99.5 (3.97) | 102 (4.11) |
| PFBS | 93.4 (12.3) | 107 (6.18) | 91.8 (7.88) | 100 (5.38) | 105 (6.90) |
| PFPeS | 102 (13.3) | 113 (8.62) | 90.2 (4.13) | 99.7 (2.68) | 103 (4.89) |
| PFHxS | 89.3 (17.7) | 95.2 (8.96) | 100 (12.7) | 98.6 (6.64) | 105 (6.83) |
| PFHpS | 85.1 (16.1) | 109 (9.59) | 96.0 (7.89) | 106 (3.56) | 111 (2.42) |
| PFOS | 79.0 (18.5) | 107 (6.22) | 86.1 (8.60) | 101 (7.45) | 110 (4.62) |
| PFNS | 104 (9.28) | 92.5 (14.2) | 85.0 (9.21) | 98.9 (4.69) | 108 (3.95) |
| PFDS | 109 (11.1) | 106 (11.0) | 92.6 (13.1) | 101 (7.80) | 101 (4.53) |
| PFUdS | 95.6 (18.8) | 102 (8.93) | 77.9 (7.72) | 90.2 (8.96) | 96.4 (7.42) |
| PFDoS | 111 (9.06) | 95.0 (10.7) | 80.0 (13.7) | 83.1 (8.45) | 99.5 (12.4) |
| PFTrDS | 94.1 (14.9) | 107 (15.1) | 79.8 (12.5) | 85.2 (12.4) | 96.4 (15.1) |
| 4:2 FTS | 122 (5.65) | 113 (4.55) | 91.5 (7.23) | 100 (3.92) | 104 (5.01) |
| 6:2 FTS | 110 (10.3) | 109 (12.0) | 93.1 (10.0) | 95.2 (7.65) | 102 (5.42) |
| 8:2 FTS | 116 (10.0) | 102 (16.1) | 89.3 (12.6) | 101 (5.81) | 106 (3.58) |
| 3:3 FTCA | – | – | – | – | – |
| 5:3 FTCA | – | 115 (7.21) | 104 (8.42) | 108 (7.38) | 108 (5.05) |
| 7:3 FTCA | – | 122 (6.51) | 92.6 (18.9) | 99.7 (9.76) | 105 (5.88) |
| FOSA | 110 (8.60) | 101 (10.9) | 79.1 (4.69) | 89.4 (5.01) | 96.6 (6.21) |
| NMeFOSA | – | – | 110 (18.8) | 92.1 (10.3) | 116 (3.63) |
| NEtFOSA | – | – | 116 (9.55) | 103 (11.9) | 104 (11.6) |
| NMeFOSAA | – | 118 (10.3) | 98.2 (12.1) | 98.8 (9.01) | 100 (6.96) |
| NEtFOSAA | – | – | 106 (10.9) | 96.1 (11.1) | 92.6 (5.99) |
| PFMPA | 94.3 (11.0) | 105 (7.54) | 88.4 (7.38) | 98.1 (3.33) | 102 (4.66) |
| PFMBA | 85.9 (7.17) | 99.9 (10.5) | 87.0 (8.69) | 101 (4.18) | 109 (5.75) |
| NFDHA | – | – | – | 90.5 (13.9) | 114 (3.75) |
| HFPO-DA | – | – | 111 (6.49) | 92.5 (16.2) | 95.6 (12.4) |
| ADONA | 90.8 (12.6) | 116 (5.19) | 90.9 (4.78) | 103 (3.45) | 106 (5.37) |
| PFEESA | 89.4 (12.7) | 113 (8.74) | 92.8 (1.95) | 102 (1.80) | 108 (3.07) |
| 9Cl-PF3ONS | 95.4 (10.3) | 102 (9.40) | 85.4 (6.64) | 101 (4.97) | 106 (4.67) |
| 11Cl-PF3OUdS | 95.1 (8.68) | 105 (3.84) | 82.7 (4.07) | 97.5 (7.82) | 101 (6.36) |
Measurement of 45 Targeted PFAS in Potable and Non-Potable Waters
Each sample was prepared in triplicate with the addition of EIS. Consistent with the accuracy and precision analysis, the recoveries of EIS were within 30% of the nominal concentration (100 ppt) across all source waters. This demonstrated that the established method was suitable for accurate measurement of targeted PFAS, including ultrashort-chain PFAS, in a wide range of water matrices (Table VI).
Table VI: Measurement of 45 Targeted PFAS in Various Water Matrices (nd = non-detected)
| Water Samples | Averaged Concentration (ng/L) | ||||||||
| Ultrashort-Chain | Short-Chain | ||||||||
| TFA | PFPrA | TFMS | PFBA | PFBS | PFPeA | PFHxA | PFHxS | PFHpA | |
| Potable Waters | |||||||||
| Tap Water #1 | 176 | 2.66 | 8.40 | nd | nd | <1.00 | 2.13 | nd | nd |
| Tap Water #2 | 317 | 1.54 | 7.14 | nd | 2.25 | nd | nd | nd | nd |
| Tap Water #3 | 166 | 3.15 | 10.9 | nd | 2.14 | 2.51 | 1.24 | nd | nd |
| Tap Water #4 | 330 | 5.48 | 12.8 | nd | 1.68 | 1.97 | nd | nd | nd |
| Tap Water #5 | 1186 | 29.0 | 25.4 | 13.2 | 8.58 | 13.7 | 17.7 | nd | 5.78 |
| Bottled Water #1 (spring water) | 107 | nd | nd | nd | nd | nd | nd | nd | nd |
| Bottled Water #2 (spring water) | 257 | 1.66 | <1.00 | <2.00 | 1.21 | nd | nd | nd | nd |
| Bottled Water #3 (RO purified) | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Natural Spring Water | 486 | 2.91 | 4.81 | nd | nd | nd | nd | nd | nd |
| Well Water #1 | 557 | 1.82 | 1.74 | nd | nd | nd | nd | nd | nd |
| Well Water #2 | 171 | 1.16 | <1.00 | nd | nd | nd | nd | nd | nd |
| Non-Potable Waters | |||||||||
| Creek Water #1 | 605 | 4.88 | 6.75 | 5.82 | 19.5 | 3.50 | 1.52 | nd | <1.00 |
| Creek Water #2 | 626 | 3.07 | 2.26 | nd | 2.68 | nd | nd | nd | nd |
| Creek Water #3 | 627 | 10.9 | 8.28 | <2.00 | 2.19 | 4.94 | 2.35 | nd | nd |
| POTW Water Effluent | 1156 | 24.2 | 9.66 | nd | 2.84 | 6.50 | 24.0 | 2.28 | <1.00 |
| Hospital Effluent | 487 | 3.08 | 1.45 | nd | nd | 2.15 | 1.50 | nd | nd |
| Metal Finisher Effluent | 678 | 2.78 | 8.89 | 2.87 | 3.11 | 2.52 | 2.09 | nd | nd |
| Chemical Manufacture Effluent | 90,473 | 8,540 | 13.3 | 62.8 | 1.13 | 100 | 7.22 | nd | 12.0 |
| Water Samples | Averaged Concentration (ng/L) | |||
| Long-Chain | Alternatives | |||
| PFOA | PFOS | PFMPA | HFPO-DA | |
| Potable Waters | ||||
| Tap Water #1 | <1.00 | nd | nd | nd |
| Tap Water #2 | nd | <2.00 | nd | nd |
| Tap Water #3 | <1.00 | 4.76 | nd | nd |
| Tap Water #4 | nd | nd | nd | nd |
| Tap Water #5 | 18.0 | 5.63 | nd | nd |
| Bottled Water #1 (spring water) | nd | nd | nd | nd |
| Bottled Water #2 (spring water) | nd | <2.00 | nd | nd |
| Bottled Water #3 (RO purified) | nd | nd | nd | nd |
| Natural Spring Water | nd | nd | nd | nd |
| Well Water #1 | <1.00 | nd | nd | nd |
| Well Water #2 | nd | nd | nd | nd |
| Non-Potable Waters | ||||
| Creek Water #1 | <1.00 | 6.06 | 4.40 | nd |
| Creek Water #2 | nd | <2.00 | nd | nd |
| Creek Water #3 | 3.08 | 5.97 | nd | nd |
| POTW Water Effluent | 12.9 | 3.88 | nd | nd |
| Hospital Effluent | nd | nd | nd | nd |
| Metal Finisher Effluent | <1.00 | 112 | nd | nd |
| Chemical Manufacture Effluent | 20.8 | nd | 14.0 | 5,709 |
Conclusions
A simple and reliable dilute-and-shoot workflow was established in this study to provide a unique solution that incorporates ultrashort-chain PFAS into a comprehensive analysis of PFAS in various water matrices. Use of an Ultra Inert IBD column, which is a polar-embedded alkyl phase column with an inert coating on the hardware, ensured that the method was sensitive, accurate, and precise. Most important, this method can serve as a valuable tool for monitoring these emergent PFAS in environmental water systems and assist in generating guidelines for future regulatory actions. Visit www.restek.com/PFAS for additional products, methods, and technical resources supporting PFAS analysis.
References
- B.F. Scott, C.A. Moody, C. Spencer, J.M. Small, D.C.G. Muir, S.A. Mabury, Analysis of perfluorocarboxylic acids/anions in surface waters and precipitation using GC-MS and analysis of PFOA from large volume samples, Environ. Sci. Technol. 40 (2006) 6405–6410, https://doi.org/10.1021/es061131o
- S. Taniyasu, K. Kannan, L.W.Y. Yeung, K.Y. Kwok, P.K.S. Lam, N. Yamashita, Analysis of trifluoroacetic acid and other short-chain perfluorinated acids (C2-C4) in precipitation by liquid chromatography-tandem mass spectrometry: comparison to patterns of long-chain perfluorinated acids (C5-C18), Anal. Chim. Acta. 619 (2008) 221–230, https://doi.org/10.1016/j.aca.2008.04.064
- S.-H. Liang, J.A. Steimling, M. Chang, Analysis of ultrashort-chain and short-chain (C1 to C4) per- and polyfluorinated substances in potable and non-potable waters, J. Chromatogr. Open 4 (2023) 100098, https://doi.org/10.1016/j. jcoa.2023.100098
- S.-H. Liang, J.A. Steimling, Integration of ultrashort-chain compounds into the biomonitoring of per- and polyfluorinated substances in human plasma and serum, J. Chromatogr. Open 5 (2024) 100132, https://doi.org/10.1016/j. jcoa.2024.100132