Key Highlights
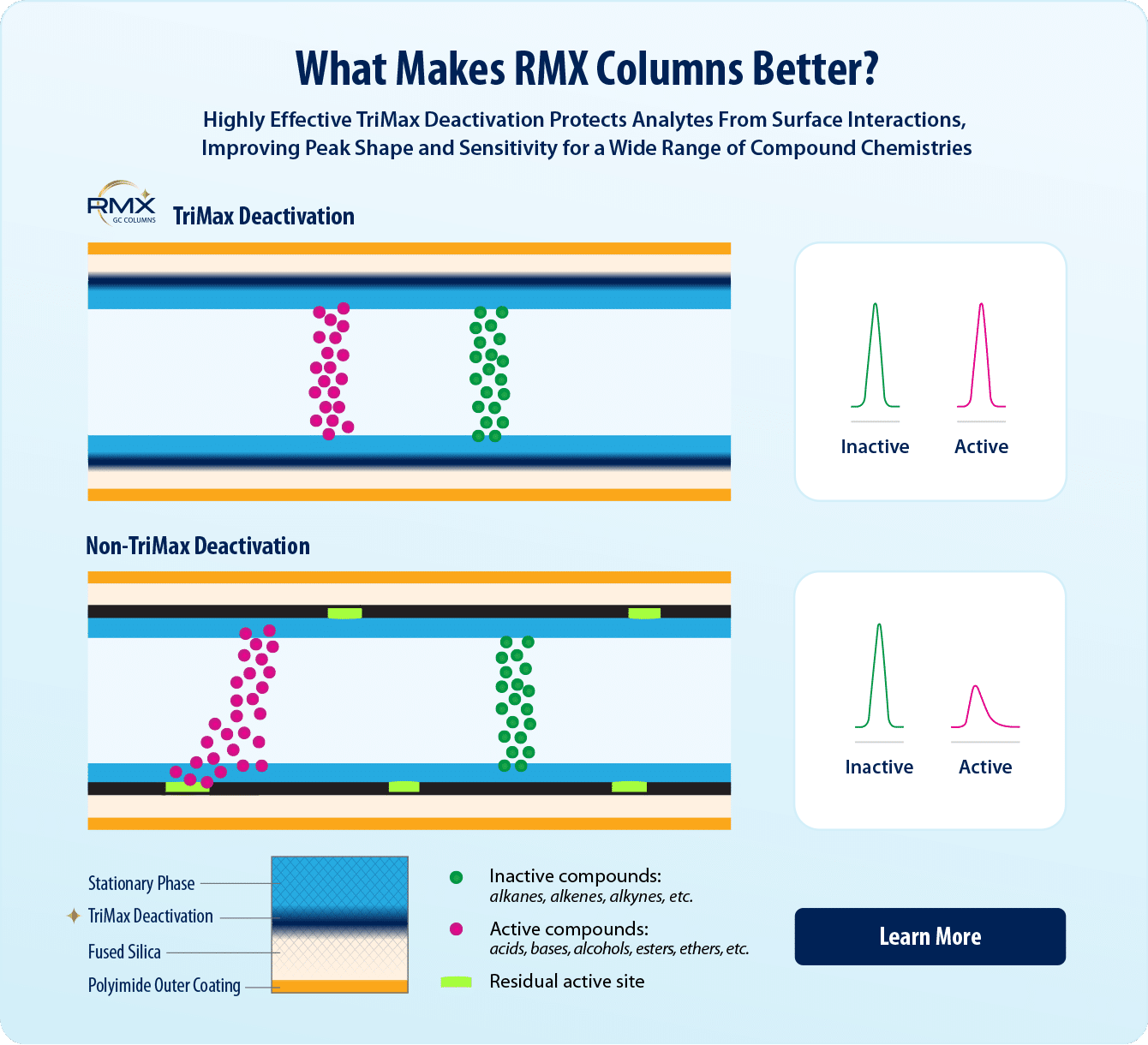
- Highly effective TriMax column deactivation produces an exceptionally inert sample flow path for acids, bases, and neutrals.
- Maximum inertness results in maximum sensitivity for a wide range of challenging semivolatiles.
- Compared to a premium competitor column, the RMX-5Sil MS column had a lower MDL for 60% and lower LLOQ for 63% of the 52 compounds tested.

Abstract
In this study, we examined the impact of GC column deactivation on lowering detection limits for semivolatiles analysis when using GC-MS/MS. The MDLs and LLOQs generated using an RMX-5Sil MS column were found to be significantly lower for approximately two thirds of the compounds tested compared to results obtained on a premium competitor column. Better sensitivity was achieved for a wide range of compound chemistries due to the more effective deactivation used in RMX-5Sil MS columns.
Introduction
Accurate quantification of semivolatile organic compounds at trace levels in environmental matrices is critical for assessing contamination, regulatory compliance, and risks to human and ecological health. GC-MS/MS is a cornerstone technique for lowering detection and quantitation limits for semivolatiles due to its high selectivity and sensitivity. Greater sensitivity allows labs to adopt alternative sample preparation methods that scale down extraction volumes and thereby reduce the use of use of chlorinated solvents. Method sensitivity is typically characterized by two key parameters: the method detection limit (MDL) and the lower limit of quantitation (LLOQ). The MDL represents the lowest concentration that can be distinguished from method blank results with 99% confidence. In contrast, the LLOQ defines the lowest concentration at which a laboratory demonstrates that an analyte can be accurately quantified.
Lowering the MDL and LLOQ in GC-MS/MS semivolatiles methods involves optimizing multiple factors, including sample preparation, injection techniques, chromatographic conditions, and mass spectrometer parameters. In addition, the inertness of the sample flow path plays a critical role in lowering detection and quantitation limits for semivolatiles. GC column manufacturers use different deactivation processes to neutralize the column surface and prevent interactions that reduce sensitivity through unstable peak shapes, but traditional deactivations tend to work better for some compound classes than others. Restek has developed a next-generation TriMax deactivation used on all RMX columns that creates an exceptionally inert surface that is effective across a wide range of semivolatile compound chemistries.
This study compares the detection and quantitation limits that could be achieved with an RMX-5Sil MS column compared to another manufacturer’s premium column by assessing MDL and LLOQ levels. Experiments were conducted using solvent-based standards instead of matrix in order to evaluate column performance specifically without the impact of sample handling and extraction.
Experimental
Standard Preparation
Calibration standards were prepared in methylene chloride at 0.5, 1, 2, 5, 10, 20, 50, 100, 200, 500, 1000, 2000, and 5000 ppb. For both columns, calibration curves were run on day 1 along with triplicate injections of the 0.5-100 ppb standards. The linear range of the calibration curve was determined for each semivolatile based on compound response. On days 2 and 3, fresh 0.5-100 ppb standards were prepared and injected in triplicate and used to determine the MDL and LLOQ for each semivolatile on each column.
Instrument Conditions
Samples were run on an RMX-5Sil MS column and a competitor’s premium column in a 30 m, 0.25 mm ID, 0.25 μm format. A Thermo TRACE 1310 GC paired with a TSQ 8000 mass spectrometer was used for semivolatiles analysis under the conditions listed below.
Injection volume: 1 µL
Liner: Topaz 4 mm Precision inlet liner with wool (cat.# 23267)
Injection port: 280 °C; 10:1 split; 1.2 mL/min
Carrier gas: helium
Oven: 40 °C (hold 1 min) to 280 °C at 12 °C/min to 310 °C at 3 °C/min
Detector: MS/MS; SRM mode; 280 °C transfer line temp; 330 °C source (see chromatogram for SRM transitions)

Data Analysis
MDLs were calculated for each compound on each column by multiplying the standard deviation of the recalculated amount of its lowest calibration point by t=2.896 (n=9). LLOQ was determined from the same samples and defined as the lowest calibration point with a recovery between 80-120% (or closest % recovery available) for each semivolatile on each column.
Results and Discussion
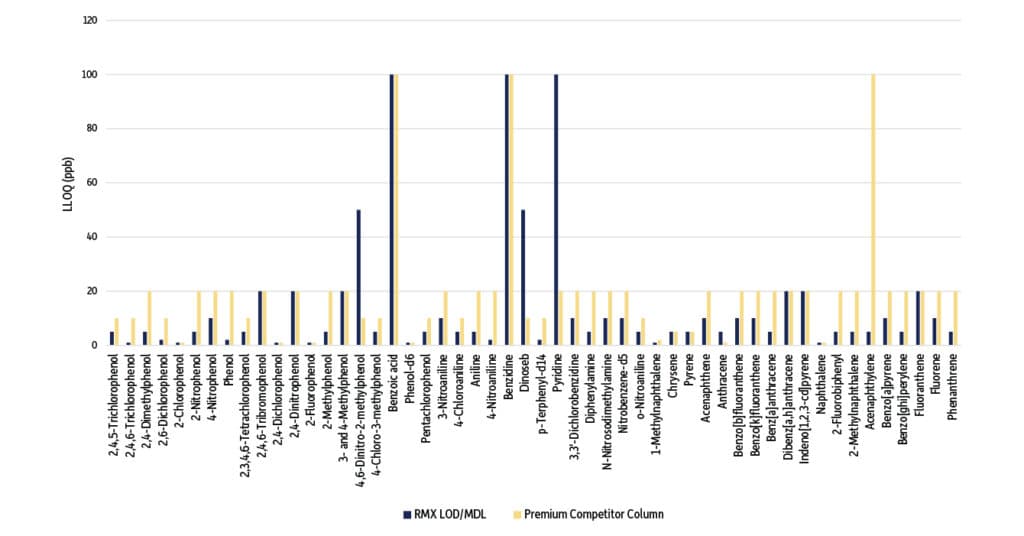
The MDL was lower on the RMX 5Sil MS column for 60% (31/52) of the compounds evaluated (Table I, Figure 1). Similarly, the LLOQ was lower on the RMX 5Sil MS column for 63% (33/52) of the target analytes (Table I, Figure 2). Individual results for each compound are presented in Table II. Lower MDL and LLOQ values mean that greater sensitivity can be achieved on the RMX-5Sil MS column, which can be attributed to the superior inertness produced by Restek’s new surface deactivation technology. Notably, lower detection limits were achieved for a wide range of compound classes, including acidic, basic, and neutral semivolatiles when using the RMX-5Sil MS column.
Table I: Overall, highly inert RMX-5Sil MS columns produced lower LLOQs and MDLs for more compounds than a competitor’s premium column, allowing lower detection limits for semivolatiles analysis.
| LLOQ (ppb) | LLOQ (ppb) | LLOQ (ppb) | MDL (ppb | MDL (ppb) | MDL (ppb | |||
|---|---|---|---|---|---|---|---|---|
| Column | Average | Min | Max | RMX-5Sil Has Best Performance | Average | Min | Max | RMX-5Sil Has Best Performance |
| RMX-5Sil MS | 14 | 1 | 100 | 33/52 compounds (63%) | 1 | 0.1 | 14 | 31/52 compounds (60%) |
| Premium Competitor Column | 19 | 1 | 100 | 2 | 0.1 | 51 |
Table II: LOD and MDL Results for Individual Semivolatiles
| RMX-5Sil MS | RMX-5Sil MS | Premium Competitor Column | Premium Competitor Column | |
| Compound | LLOQ | MDL | LLOQ | MDL |
| Acenaphthylene | 5 | 0.42 | 100 | 0.60 |
| Phenol | 2 | 0.45 | 20 | 0.27 |
| 4-Nitroaniline | 2 | 0.22 | 20 | 0.43 |
| 2,4-Dimethylphenol | 5 | 0.33 | 20 | 0.30 |
| 2-Nitrophenol | 5 | 0.84 | 20 | 0.51 |
| 2-Methylphenol | 5 | 0.84 | 20 | 2.37 |
| Aniline | 5 | 0.67 | 20 | 0.43 |
| Diphenylamine | 5 | 0.74 | 20 | 0.85 |
| Benz[a]anthracene | 5 | 0.29 | 20 | 0.49 |
| 2-Fluorobiphenyl | 5 | 0.20 | 20 | 0.30 |
| 2-Methylnaphthalene | 5 | 0.15 | 20 | 0.32 |
| Benzo[ghi]perylene | 5 | 0.39 | 20 | 0.08 |
| Phenanthrene | 5 | 0.19 | 20 | 0.15 |
| 4-Nitrophenol | 10 | 1.38 | 20 | 1.31 |
| 3-Nitroaniline | 10 | 1.40 | 20 | 1.33 |
| 3,3′-Dichlorobenzidine | 10 | 1.37 | 20 | 1.76 |
| N-Nitrosodimethylamine | 10 | 0.63 | 20 | 0.53 |
| Nitrobenzene-d5 | 10 | 0.13 | 20 | .74 |
| Acenaphthene | 10 | 0.93 | 20 | 1.53 |
| Benzo[b]fluoranthene | 10 | 0.71 | 20 | 1.31 |
| Benzo[k]fluoranthene | 10 | 0.67 | 20 | 2.65 |
| Benzo[a]pyrene | 10 | 1.08 | 20 | 2.63 |
| Fluorene | 10 | 0.48 | 20 | 1.54 |
| 2,4,6-Trichlorophenol | 1 | 0.40 | 10 | 0.16 |
| 2,6-Dichlorophenol | 2 | 0.30 | 10 | 0.21 |
| p-Terphenyl-d14 | 2 | 0.30 | 10 | 0.36 |
| 2,4,5-Trichlorophenol | 5 | 0.92 | 10 | 0.33 |
| 2,3,4,6-Tetrachlorophenol | 5 | 1.52 | 10 | 1.48 |
| 4-Chloro-3-methylphenol | 5 | 0.06 | 10 | 0.21 |
| Pentachlorophenol | 5 | 0.18 | 10 | 0.93 |
| 4-Chloroaniline | 5 | 0.55 | 10 | 0.32 |
| o-Nitroaniline | 5 | 0.49 | 10 | 0.36 |
| 1-Methylnaphthalene | 1 | 0.13 | 2 | 0.05 |
| 2-Chlorophenol | 1 | 0.55 | 1 | 0.47 |
| 2,4,6-Tribromophenol | 20 | 1.79 | 20 | 3.79 |
| 2,4-Dichlorophenol | 1 | 0.32 | 1 | 0.41 |
| 2,4-Dinitrophenol | 20 | 1.94 | 20 | 2.34 |
| 2-Fluorophenol | 1 | 0.20 | 1 | 0.20 |
| 3- and 4-Methylphenol | 20 | 0.59 | 20 | 0.69 |
| Benzoic acid | 100 | 14.47 | 100 | 50.54 |
| Phenol-d6 | 1 | 0.29 | 1 | 0.32 |
| Benzidine | 100 | 0.89 | 100 | 0.97 |
| Chrysene | 5 | 0.30 | 5 | 0.23 |
| Pyrene | 5 | 103 | 5 | 0.10 |
| Dibenz[a,h]anthracene | 20 | 1.06 | 20 | 2.58 |
| Indeno[1,2,3-cd]pyrene | 20 | 0.59 | 20 | 1.81 |
| Naphthalene | 1 | 0.18 | 1 | 0.35 |
| Fluoranthene | 20 | 0.16 | 20 | 0.80 |
| Anthracene | 5 | 0.86 | 1 | 0.45 |
| 4,6-Dinitro-2-methylphenol | 50 | 1.59 | 10 | 1.80 |
| Dinoseb | 50 | 2.23 | 10 | 3.39 |
| Pyridine | 100 | 7.73 | 20 | 11.39 |
Figure 1: MDL Comparison of Semivolatiles on an RMX-5Sil MS Column vs. a Premium Competitor Column (Compounds are sorted by groups of acids, bases, and neutrals.)
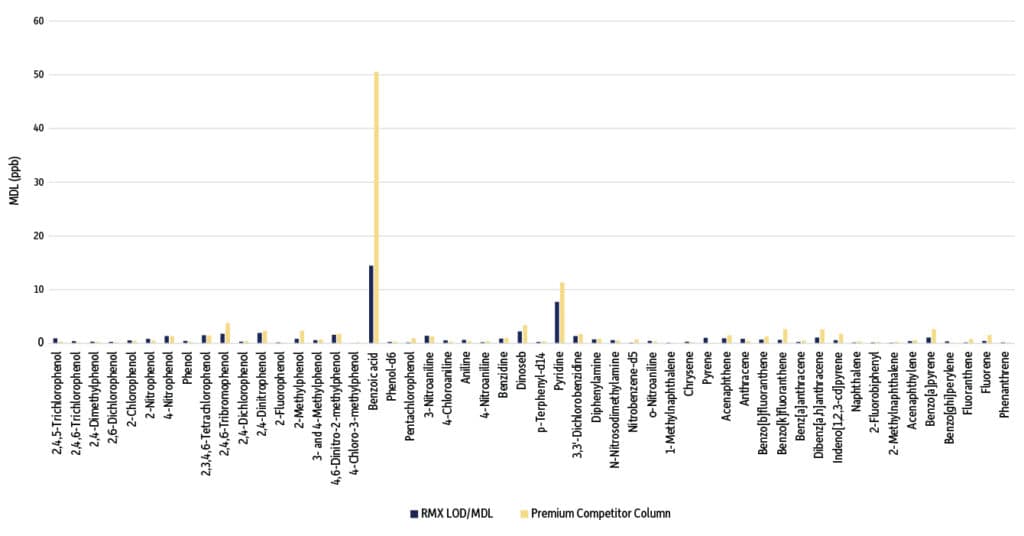
Figure 2: LLOQ Comparison of Semivolatiles on an RMX-5Sil MS Column vs. a Premium Competitor Column (Compounds are sorted by groups of acids, bases, and neutrals.)
Conclusion
An inert sample flow path allows for lower detection limits for semivolatiles, maximizing the high sensitivity that can be achieved with GC-MS/MS instruments. The work summarized here shows that exceptionally inert RMX-5Sil MS columns provide greater sensitivity than a competitor’s premium column. Assessments of both MDL and LLOQ showed that lower limits of detection and quantification could be achieved for a wide range of compound chemistries on the RMX-5Sil MS column.