- Excellente séparation des composés et temps d’analyse rapides.
- Polyvalente et applicable à tout type d’analyse de résidus d’antibiotiques — et optimisation possible pour la quantification individuelle de panels de différentes classes :
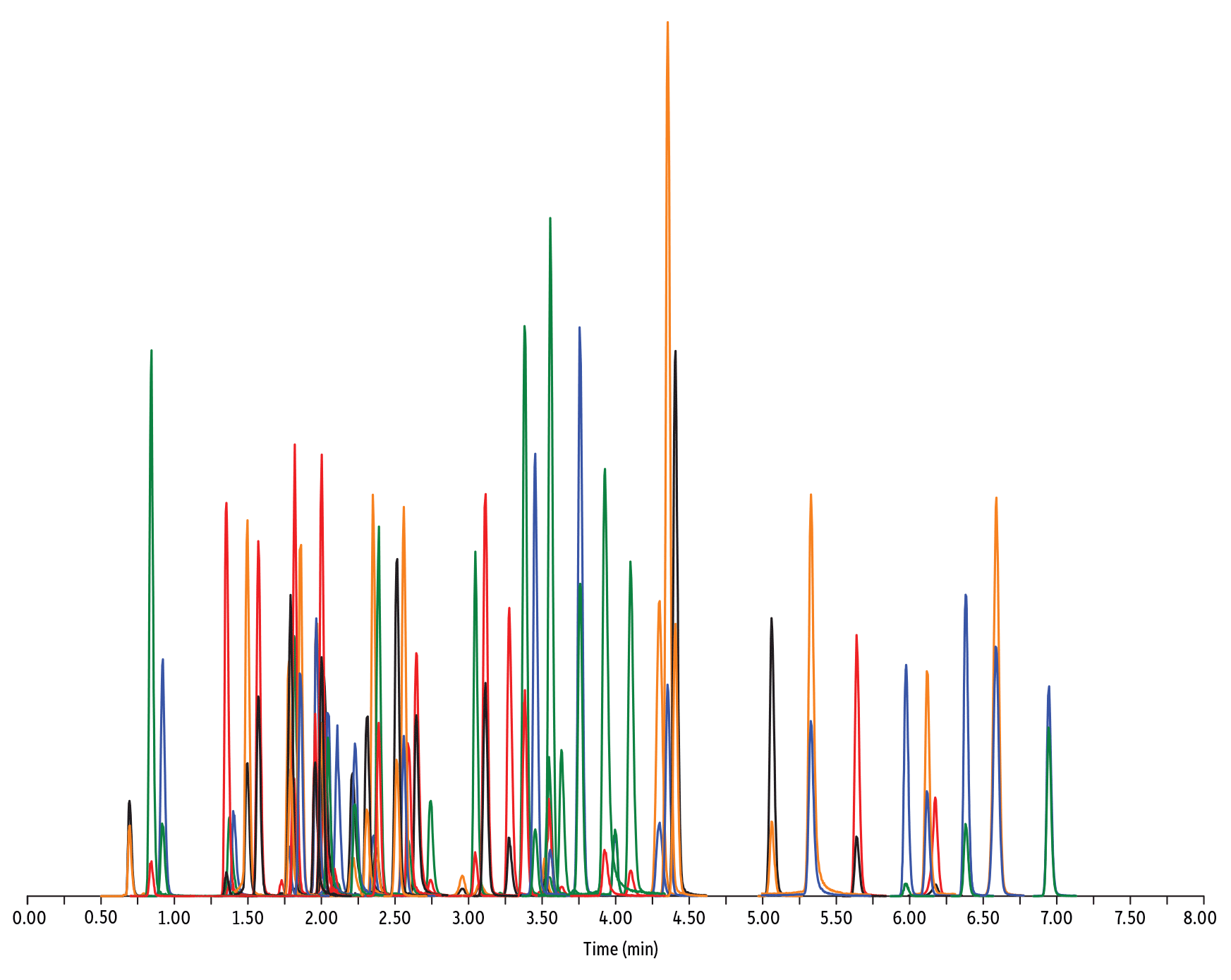
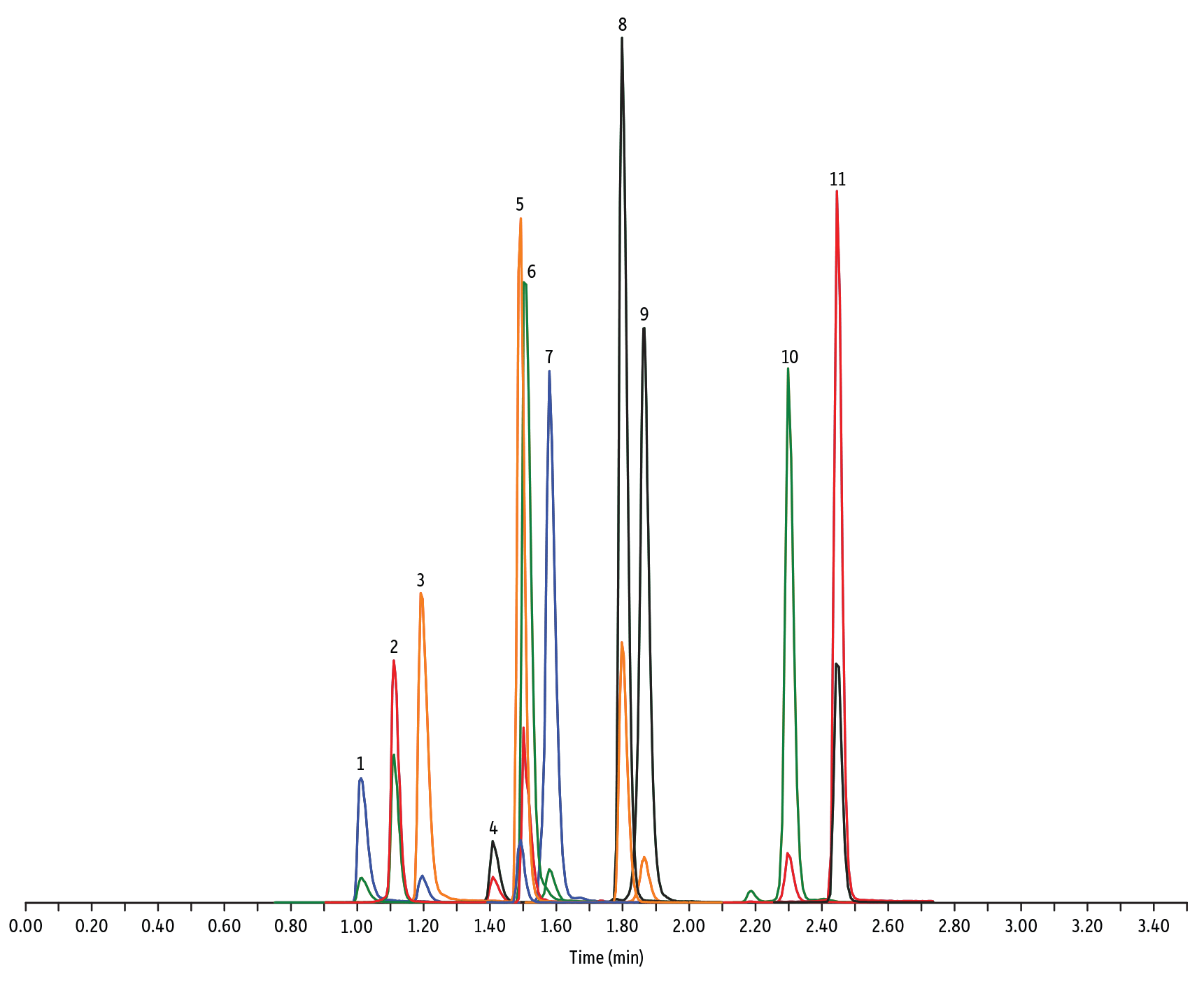
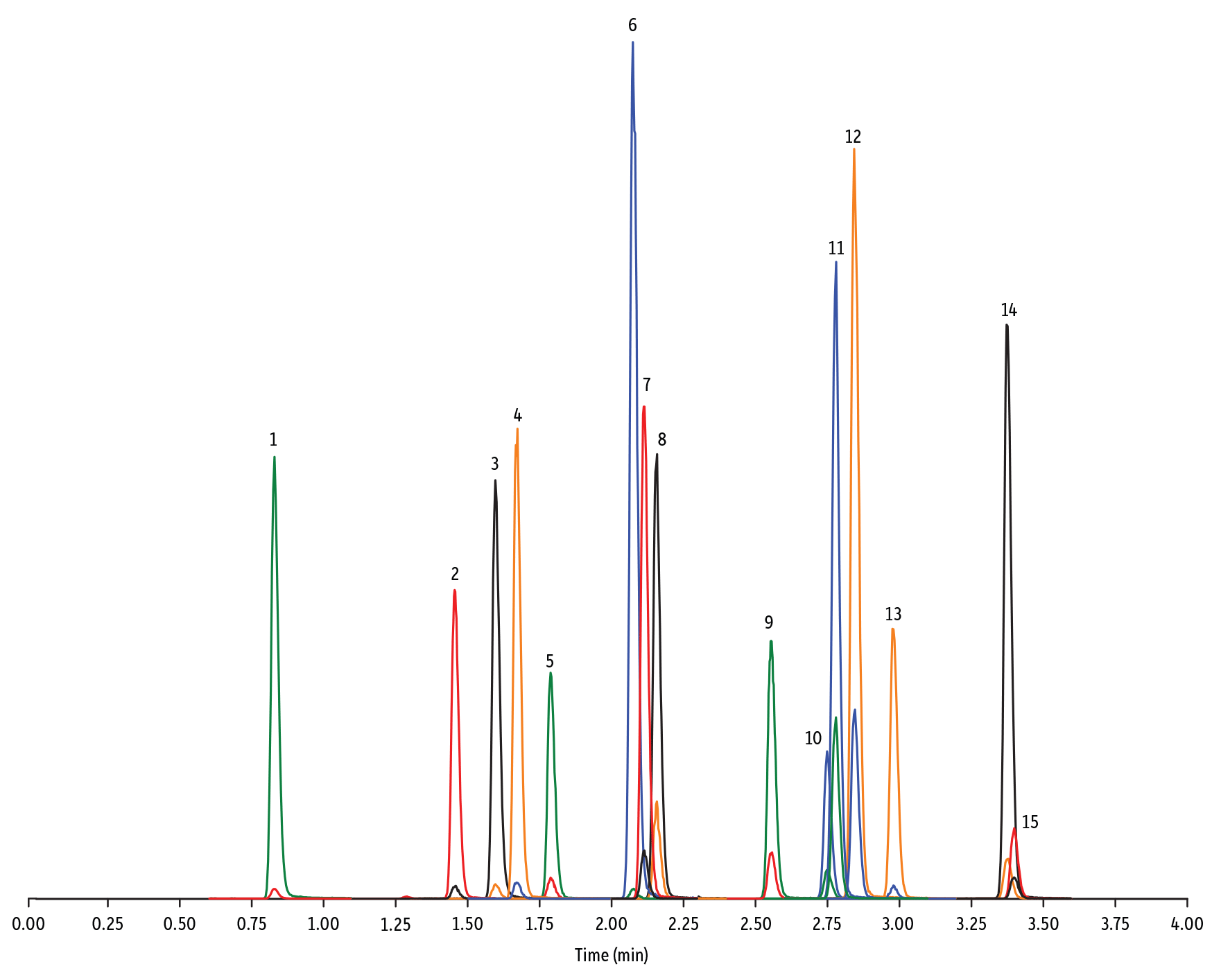
- Macrolide, Lincosamide, et Streptogramine (Figure 1)
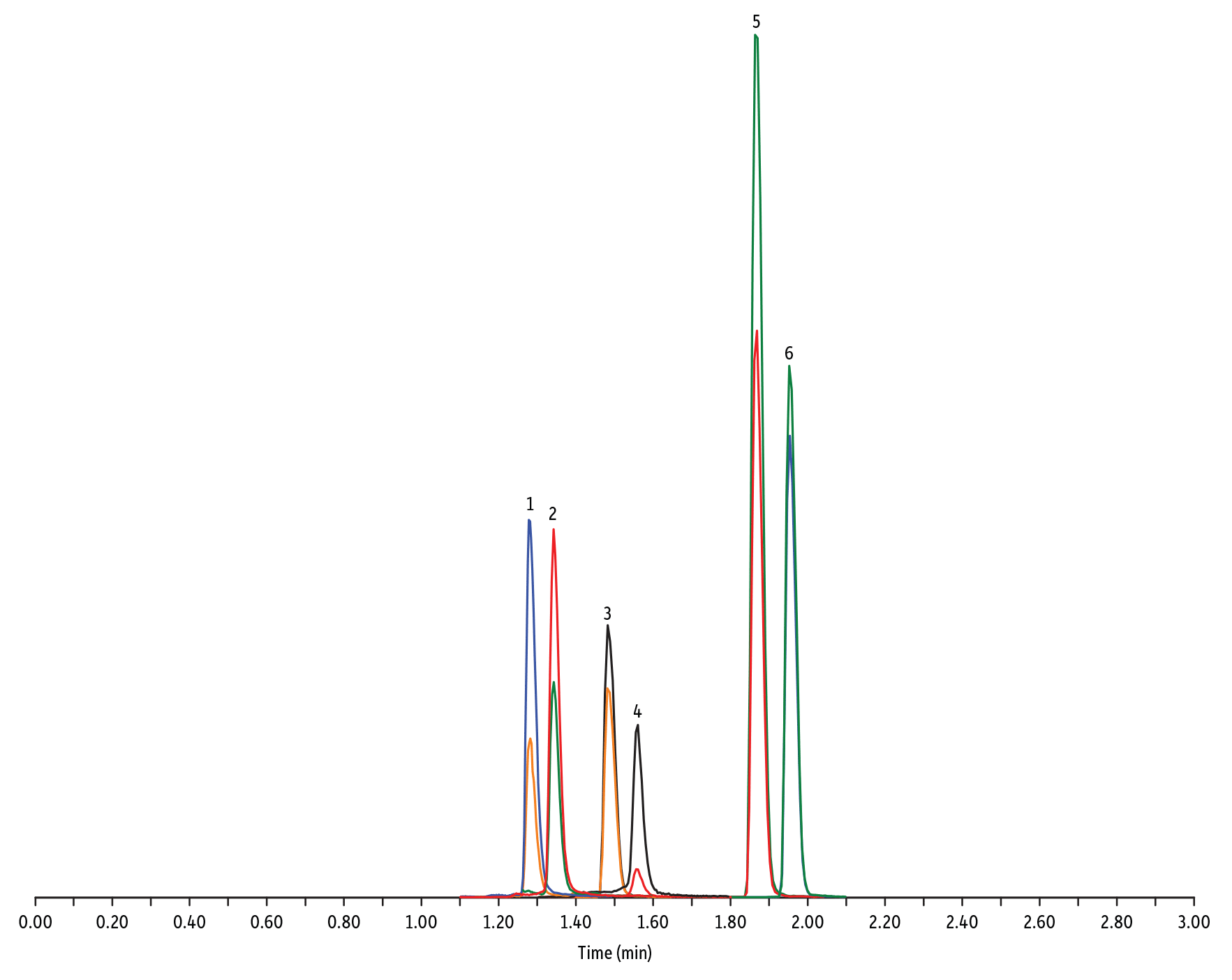
- Amphénicol et Tétracycline (Figure 2)
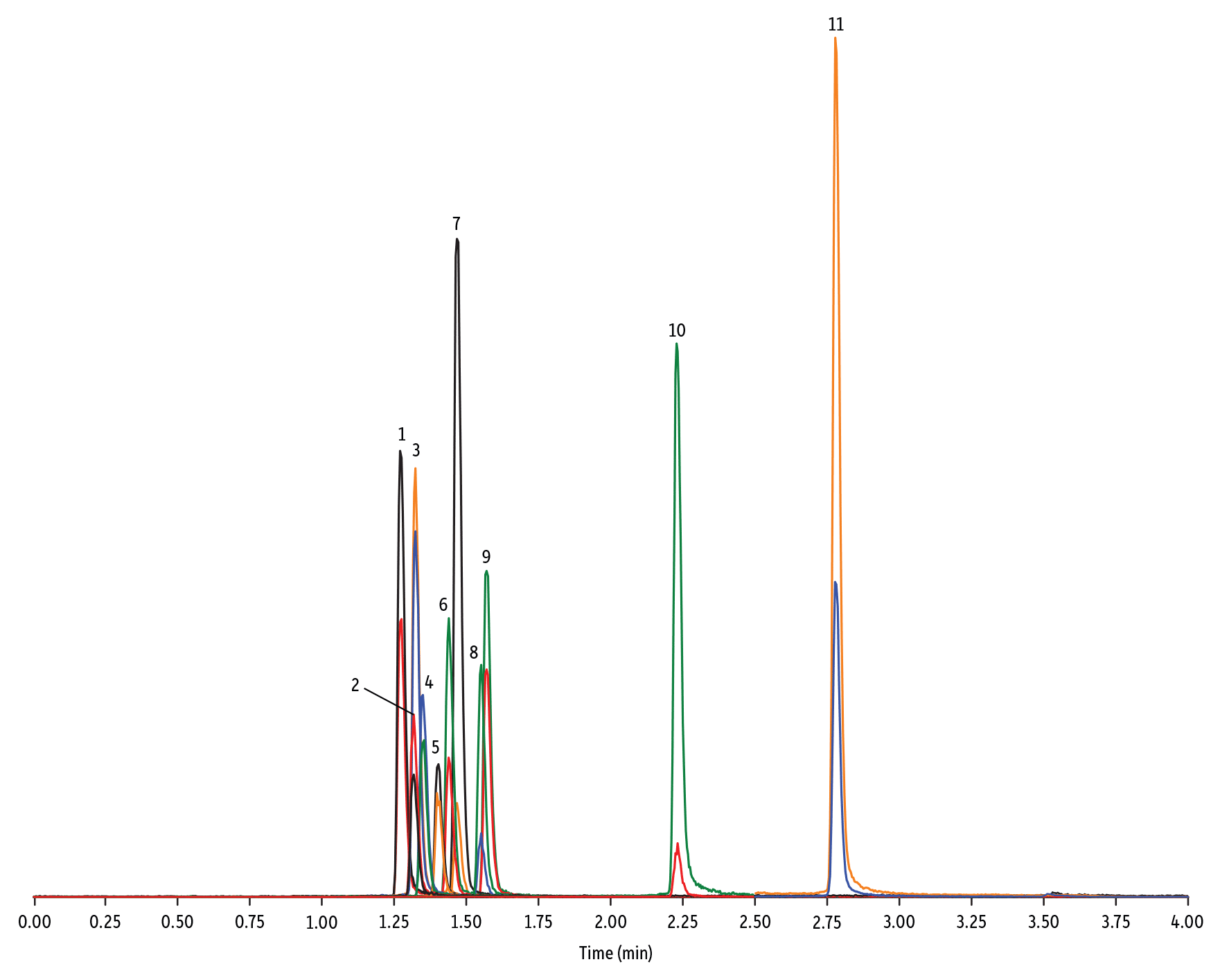
- Quinolone (Figure 3)
- Pénicilline, Céphalosporine, et Tétracycline (Figure 4)
- Sulfonamide (Figure 5) (pour les ionophores, utiliser la colonne Raptor Biphenyl. [Figure 6])
L’utilisation d’antibiotiques sur les animaux élevés pour la consommation humaine est une préoccupation de Santé Publique et de sécurité en raison de la génération potentielle de bactéries résistantes aux médicaments. De nombreux pays dans l’Union Européenne ainsi que le Canada ont interdit l’utilisation d’antibiotiques à des fins non thérapeutiques, et les États-Unis définissent une politique visant à réduire l’utilisation d’antibiotiques médicalement importants pour stimuler la croissance. Afin de réglementer l’usage approprié des antibiotiques vétérinaires, la FDA aux États-Unis a fixé des limites maximales pour les résidus (MRL – “Maximum Residue Limits”) pour différents tissus animaux et produits alimentaires (21 CFR Part 556). Une méthode analytique sensible, efficace et fiable pour différentes classes d’antibiotiques est donc nécessaire pour répondre à ces exigences réglementaires, et la colonne LC Restek Raptor C18 est la colonne idéale pour cela.
LC_FS0506
Peaks
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 1. | Desacetyl cephapirin | 0.70 | 150 | 382.03 | 111.92 | 124.21 |
| 2. | Sulfanilamide | 0.85 | 200 | 172.98 | 93.07 | 75.23 |
| 3. | Amoxicillin | 0.92 | 100 | 366.24 | 349.10 | 208.07 |
| 4. | Cephapirin | 1.36 | 50 | 424.17 | 292.08 | 124.14 |
| 5. | Tildipirosin | 1.38 | 200 | 734.59 | 561.45 | 204.15 |
| 6. | Desfuroyl ceftiofur cysteine disulfide | 1.40 | 300 | 549.16 | 183.02 | 126.00 |
| 7. | Lincomycin | 1.50 | 50 | 407.32 | 359.23 | 389.28 |
| 8. | Sulfadiazine | 1.57 | 20 | 251.18 | 156.04 | 92.08 |
| 9. | Cefquinome | 1.73 | 200 | 529.19 | 134.10 | 125.12 |
| 10. | Ampicillin | 1.78 | 50 | 350.19 | 106.07 | 160.06 |
| 11. | Sulfathiazole | 1.79 | 10 | 256.16 | 156.03 | 92.08 |
| 12. | Marbofloxacin | 1.81 | 10 | 363.20 | 72.11 | 320.10 |
| 13. | Cefalexin | 1.82 | 100 | 348.10 | 158.05 | 174.05 |
| 14. | Sulfapyridine | 1.86 | 10 | 250.13 | 156.10 | 92.08 |
| 15. | Norfloxacin | 1.96 | 20 | 320.23 | 276.20 | 233.13 |
| 16. | Ofloxacin | 1.98 | 10 | 362.21 | 318.20 | 261.15 |
| 17. | Sulfamerazine | 2.00 | 20 | 265.08 | 156.03 | 92.08 |
| 18. | Cefalonium | 2.01 | 100 | 459.16 | 337.03 | 123.10 |
| 19. | Oxytetracycline | 2.02 | 25 | 461.27 | 426.15 | 443.32 |
| 20. | Ciprofloxacin | 2.04 | 20 | 332.18 | 288.22 | 245.15 |
| 21. | Cefacetrile | 2.09 | 300 | 362.07 | 258.08 | 178.01 |
| 22. | Tulathromycin A | 2.11 | 100 | 806.65 | 577.42 | 420.31 |
| 23. | Tetracycline | 2.21 | 25 | 445.28 | 154.07 | 427.32 |
| 24. | Danofloxacin | 2.23 | 20 | 358.22 | 340.16 | 314.21 |
| 25. | Enrofloxacin | 2.32 | 10 | 360.29 | 316.22 | 245.13 |
| 26. | Orbifloxacin | 2.35 | 10 | 396.22 | 352.17 | 226.12 |
| 27. | Thiamphenicol* | 2.38 | 200 | 354.16 | 290.04 | 184.98 |
| 28. | Sulfamethazine | 2.39 | 10 | 279.23 | 186.08 | 124.08 |
| 29. | Sulfamethizole | 2.52 | 10 | 271.17 | 156.02 | 108.02 |
| 30. | Sulfamethoxypyridazine | 2.56 | 10 | 281.14 | 156.03 | 126.07 |
| 31. | Sarafloxacin | 2.59 | 10 | 386.20 | 342.20 | 368.15 |
| 32. | Difloxacin | 2.65 | 10 | 400.23 | 356.17 | 299.13 |
| 33. | Cefazolin | 2.75 | 100 | 455.10 | 323.06 | 295.09 |
| 34. | Spiramycin | 2.96 | 200 | 843.64 | 540.36 | 699.48 |
| 35. | Pirlimycin | 3.05 | 20 | 411.32 | 363.18 | 327.21 |
| 36. | Chlortetracycline | 3.08 | 25 | 479.27 | 154.07 | 371.06 |
| 37. | Sulfachlorpyridazine | 3.12 | 20 | 285.05 | 156.03 | 108.09 |
| 38. | Gamithromycin | 3.28 | 100 | 777.63 | 619.52 | 601.45 |
| 39. | Sulfadoxine | 3.39 | 10 | 311.17 | 156.03 | 108.09 |
| 40. | Sulfamethoxazole | 3.46 | 20 | 254.18 | 155.98 | 147.06 |
| 41. | Cefoperazone | 3.52 | 100 | 646.26 | 143.07 | 148.02 |
| 42. | Florfenicol* | 3.55 | 200 | 356.10 | 336.02 | 184.98 |
| 43. | Sulfaethoxypyridazine | 3.56 | 20 | 295.17 | 267.07 | 156.03 |
| 44. | Tilmicosin | 3.64 | 100 | 869.72 | 696.50 | 522.42 |
| 45. | Sulfisoxazole | 3.76 | 20 | 268.14 | 156.03 | 113.10 |
| 46. | Oxolinic acid | 3.94 | 5 | 262.10 | 244.06 | 215.96 |
| 47. | Chloramphenicol* | 4.00 | 200 | 321.16 | 151.99 | 257.04 |
| 48. | Ceftiofur | 4.11 | 50 | 524.14 | 241.08 | 125.24 |
| 49. | Erythromycin | 4.31 | 25 | 734.64 | 576.40 | 558.38 |
| 50. | Sulfadimethoxine | 4.36 | 10 | 311.17 | 156.09 | 108.09 |
| 51. | Sulfaquinoxaline | 4.42 | 20 | 301.18 | 156.04 | 108.02 |
| 52. | Tylosin** | 4.67 | 100 | 916.62 | 772.49 | 598.36 |
| 53. | Penicillin G | 5.07 | 100 | 335.18 | 176.07 | 160.07 |
| 54. | Flumequine | 5.34 | 5 | 262.15 | 244.11 | 202.03 |
| 55. | Penicillin V | 5.56 | 100 | 351.10 | 160.06 | 114.07 |
| 56. | Oxacillin | 5.99 | 100 | 402.15 | 160.05 | 114.06 |
| 57. | Virginiamycin M1 | 6.13 | 50 | 526.43 | 508.31 | 355.10 |
| 58. | Tylvalosin | 6.19 | 50 | 1042.71 | 814.46 | 640.39 |
| 59. | Cloxacillin | 6.39 | 100 | 436.15 | 277.06 | 160.05 |
| 60. | Nafcillin | 6.60 | 25 | 415.19 | 199.09 | 171.06 |
| 61. | Dicloxacillin | 6.96 | 100 | 470.11 | 160.05 | 311.02 |
Conditions
| Column | Raptor C18 (cat.# 9304A12) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||
| Guard Column: | Raptor C18 EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9304A0252) | ||||||||||||||||||||||||
| Temp.: | 35 °C | ||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||
| Diluent: | Water | ||||||||||||||||||||||||
| Conc.: | 5–300 ng/mL | ||||||||||||||||||||||||
| Inj. Vol.: | 2 µL | ||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||
| A: | 0.1% Formic acid in water | ||||||||||||||||||||||||
| B: | 0.1% Formic acid in acetonitrile | ||||||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Mode: | ESI+/ESI- |
| Mode: | Scheduled MRM |
| Instrument | UHPLC |
| Notes | 1. Positive and negative polarity data were collected simultaneously from a single injection. 2. Amphenicol compounds (chloramphenicol, thiamphenicol, and florfenicol) were detected with negative polarity. 3. The MRM was scheduled at +/- 20 to 30 seconds for each analyte. 4. Multiclass antibiotics include penicillin, cephalosporin, tetracycline, sulfonamide, macrolide, lincosamide, streptogramin, amphenicol, and quinolone. **The retention time for Tylosin is noted in the peak list; however, it was not included in the chromatogram. Want even better performance when analyzing metal-sensitive compounds? Check out Inert LC columns at www.restek.com/inert. |
LC_FS0502
Peaks
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 1. | Tildipirosin | 1.01 | 200 | 734.59 | 561.45 | 204.15 |
| 2. | Lincomycin | 1.11 | 50 | 407.32 | 359.23 | 389.28 |
| 3. | Tulathromycin A | 1.19 | 100 | 806.65 | 577.42 | 420.31 |
| 4. | Spiramycin | 1.41 | 200 | 843.64 | 540.36 | 699.48 |
| 5. | Pirlymycin | 1.49 | 20 | 411.32 | 363.18 | 327.21 |
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 6. | Gamithromycin | 1.50 | 100 | 777.63 | 619.52 | 601.45 |
| 7. | Tilmicosin | 1.58 | 100 | 869.72 | 696.50 | 522.42 |
| 8. | Erythromycin | 1.80 | 25 | 734.64 | 576.40 | 558.38 |
| 9. | Tylosin | 1.87 | 100 | 916.62 | 772.49 | 598.36 |
| 10. | Tylvalosin | 2.30 | 50 | 1042.71 | 814.46 | 640.39 |
| 11. | Virginiamycin M1 | 2.45 | 50 | 526.43 | 508.31 | 355.10 |
Conditions
| Column | Raptor C18 (cat.# 9304A12) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||
| Guard Column: | Raptor C18 EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9304A0252) | ||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||
| Diluent: | Water | ||||||||||||||||||||
| Conc.: | 20–200 ng/mL | ||||||||||||||||||||
| Inj. Vol.: | 2 µL | ||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||
| A: | 0.1% Formic acid in water | ||||||||||||||||||||
| B: | 0.1% Formic acid in acetonitrile | ||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Mode: | ESI+ |
| Mode: | MRM |
| Instrument | UHPLC |
| Notes | Want even better performance when analyzing metal-sensitive compounds? Check out Inert LC columns at www.restek.com/inert. |
LC_FS0504
Peaks
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 1. | Oxytetracycline | 1.28 | 25 | 461.27 | 426.15 | 443.32 |
| 2. | Tetracycline | 1.34 | 25 | 445.28 | 154.07 | 427.32 |
| 3. | Thiamphenicol* | 1.48 | 200 | 354.16 | 290.04 | 184.98 |
| 4. | Chlortetracycline | 1.56 | 25 | 479.27 | 154.07 | 371.06 |
| 5. | Florfenicol* | 1.86 | 200 | 356.10 | 336.02 | 184.98 |
| 6. | Chloramphenicol* | 1.95 | 200 | 321.16 | 151.99 | 257.04 |
Conditions
| Column | Raptor C18 (cat.# 9304A12) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||
| Guard Column: | Raptor C18 EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9304A0252) | ||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||
| Diluent: | Water | ||||||||||||||||||||
| Conc.: | 25–200 ng/mL | ||||||||||||||||||||
| Inj. Vol.: | 2 µL | ||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||
| A: | 0.1% Formic acid in water | ||||||||||||||||||||
| B: | 0.1% Formic acid in acetonitrile | ||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Mode: | ESI+/ESI- |
| Mode: | MRM |
| Instrument | UHPLC |
| Notes | Tetracyclines and amphenicols were analyzed with ESI+ and ESI- mode, respectively. Want even better performance when analyzing metal-sensitive compounds? Check out Inert LC columns at www.restek.com/inert. |
LC_FS0505
Peaks
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 1. | Marbofloxacin | 1.28 | 10 | 363.20 | 72.11 | 320.10 |
| 2. | Norfloxacin | 1.32 | 20 | 320.23 | 276.20 | 233.13 |
| 3. | Ofloxacin | 1.32 | 10 | 362.21 | 318.20 | 261.15 |
| 4. | Ciprofloxacin | 1.35 | 20 | 332.18 | 288.22 | 245.15 |
| 5. | Danofloxacin | 1.40 | 20 | 358.22 | 340.16 | 314.21 |
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 6. | Enrofloxacin | 1.44 | 10 | 360.29 | 316.22 | 245.13 |
| 7. | Orbifloxacin | 1.47 | 10 | 396.22 | 352.17 | 226.12 |
| 8. | Sarafloxacin | 1.55 | 10 | 386.20 | 342.20 | 368.15 |
| 9. | Difloxacin | 1.57 | 10 | 400.23 | 356.17 | 299.13 |
| 10. | Oxolinic acid | 2.23 | 5 | 262.10 | 244.06 | 215.96 |
| 11. | Flumequine | 2.78 | 5 | 262.15 | 244.11 | 202.03 |
Conditions
| Column | Raptor C18 (cat.# 9304A12) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||
| Guard Column: | Raptor C18 EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9304A0252) | ||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||
| Diluent: | Water | ||||||||||||||||||||
| Conc.: | 5–20 ng/mL | ||||||||||||||||||||
| Inj. Vol.: | 2 µL | ||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||
| A: | 0.1% Formic acid in water | ||||||||||||||||||||
| B: | 0.1% Formic acid in acetonitrile | ||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Mode: | ESI+ |
| Mode: | MRM |
| Instrument | UHPLC |
| Notes | Want even better performance when analyzing metal-sensitive compounds? Check out Inert LC columns at www.restek.com/inert. |
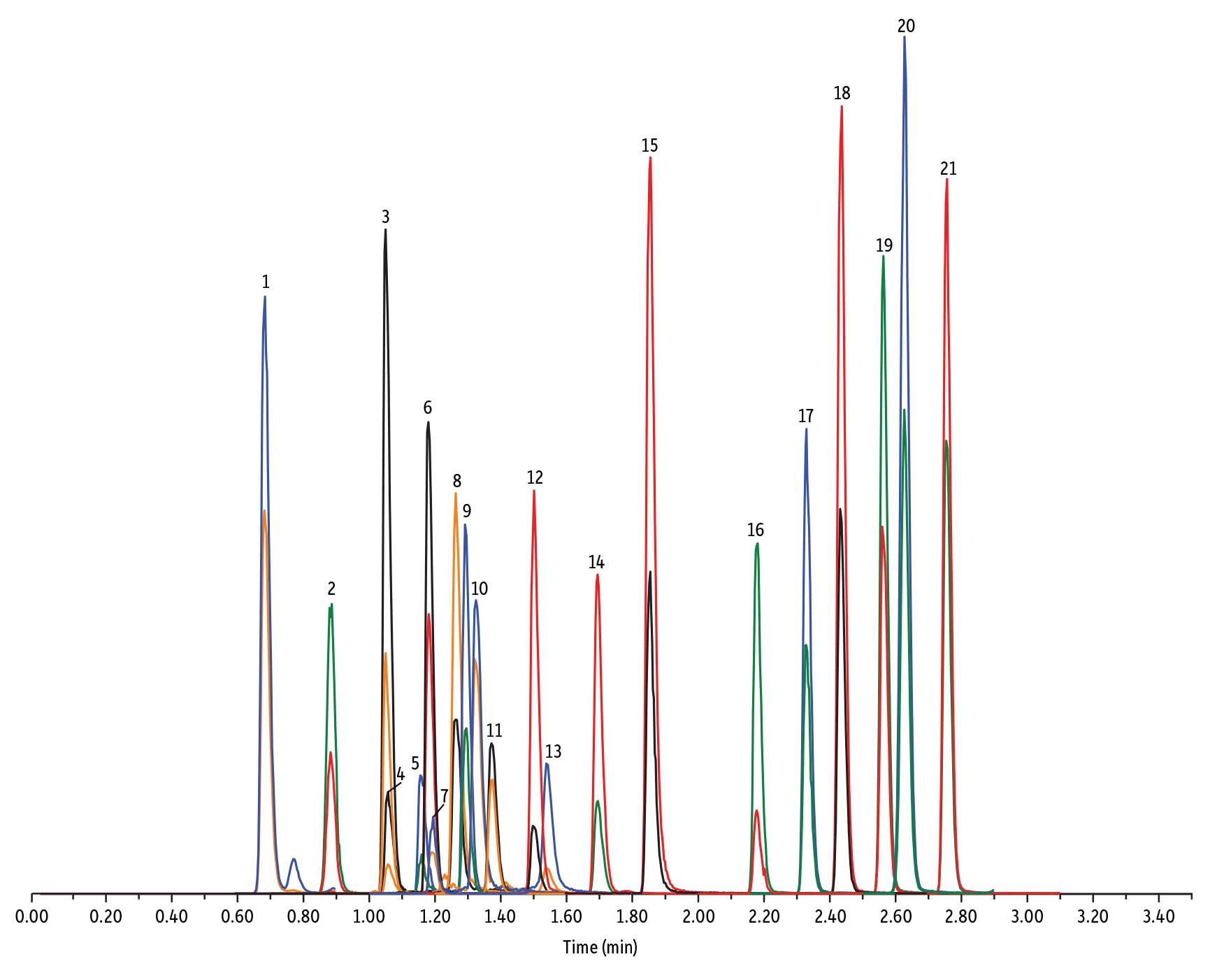
LC_FS0500
Peaks
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 1. | Desacetyl cephapirin | 0.68 | 150 | 382.03 | 111.92 | 124.21 |
| 2. | Amoxicillin | 0.89 | 100 | 366.24 | 349.10 | 208.07 |
| 3. | Cephapirin | 1.05 | 50 | 424.17 | 292.08 | 124.14 |
| 4. | Desfuroyl ceftiofur cysteine disulfide | 1.06 | 300 | 549.16 | 183.02 | 126.00 |
| 5. | Cefquinome | 1.16 | 200 | 529.19 | 134.10 | 125.12 |
| 6. | Ampicillin | 1.18 | 50 | 350.19 | 106.07 | 160.06 |
| 7. | Cefalexin | 1.19 | 100 | 348.10 | 158.05 | 174.05 |
| 8. | Oxytetracycline | 1.26 | 50 | 461.27 | 426.15 | 443.32 |
| 9. | Cefalonium | 1.29 | 100 | 459.16 | 337.03 | 123.10 |
| 10. | Tetracycline | 1.32 | 50 | 445.28 | 154.07 | 427.32 |
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 11. | Cefacetrile | 1.37 | 300 | 362.07 | 258.08 | 178.01 |
| 12. | Cefazolin | 1.50 | 100 | 455.10 | 323.06 | 295.09 |
| 13. | Chlortetracycline | 1.54 | 50 | 479.27 | 154.07 | 371.06 |
| 14. | Cefoperazone | 1.69 | 100 | 646.26 | 143.07 | 148.02 |
| 15. | Ceftiofur | 1.85 | 50 | 524.14 | 241.08 | 125.24 |
| 16. | Penicillin G | 2.18 | 100 | 335.18 | 176.07 | 160.07 |
| 17. | Penicillin V | 2.33 | 100 | 351.10 | 160.06 | 114.07 |
| 18. | Oxacillin | 2.44 | 100 | 402.15 | 160.05 | 114.06 |
| 19. | Cloxacillin | 2.56 | 100 | 436.15 | 277.06 | 160.05 |
| 20. | Nafcillin | 2.63 | 25 | 415.19 | 199.09 | 171.06 |
| 21. | Dicloxacillin | 2.76 | 100 | 470.11 | 160.05 | 311.02 |
Conditions
| Column | Raptor C18 (cat.# 9304A12) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||
| Guard Column: | Raptor C18 EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9304A0252) | ||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||
| Diluent: | Water | ||||||||||||||||||||
| Conc.: | 25–300 ng/mL | ||||||||||||||||||||
| Inj. Vol.: | 2 µL | ||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||
| A: | 0.1% Formic acid in water | ||||||||||||||||||||
| B: | 0.1% Formic acid in acetonitrile | ||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Mode: | ESI+ |
| Mode: | MRM |
| Instrument | UHPLC |
| Notes | Want even better performance when analyzing metal-sensitive compounds? Check out Inert LC columns at www.restek.com/inert. |
LC_FS0501
Peaks
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 1. | Sulfanilamide | 0.83 | 200 | 172.98 | 93.07 | 75.23 |
| 2. | Sulfadiazine | 1.45 | 20 | 251.18 | 156.04 | 92.08 |
| 3. | Sulfathiazole | 1.60 | 10 | 256.16 | 156.03 | 92.08 |
| 4. | Sulfapyridine | 1.67 | 10 | 250.13 | 156.10 | 92.08 |
| 5. | Sulfamerazine | 1.79 | 20 | 265.08 | 156.03 | 92.08 |
| 6. | Sulfamethazine | 2.07 | 10 | 279.23 | 186.08 | 124.08 |
| 7. | Sulfamethizole | 2.11 | 10 | 271.17 | 156.02 | 108.02 |
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 8. | Sulfamethoxypyridazine | 2.16 | 10 | 281.14 | 156.03 | 126.07 |
| 9. | Sulfachlorpyridazine | 2.55 | 20 | 285.05 | 156.03 | 108.09 |
| 10. | Sulfadoxine | 2.75 | 10 | 311.17 | 156.03 | 108.09 |
| 11. | Sulfamethoxazole | 2.78 | 20 | 254.18 | 155.98 | 147.06 |
| 12. | Sulfaethoxypyridazine | 2.84 | 20 | 295.17 | 267.07 | 156.03 |
| 13. | Sulfisoxazole | 2.98 | 20 | 268.14 | 156.03 | 113.10 |
| 14. | Sulfadimethoxine | 3.37 | 10 | 311.17 | 156.09 | 108.09 |
| 15. | Sulfaquinoxaline | 3.40 | 20 | 301.18 | 156.04 | 108.02 |
Conditions
| Column | Raptor C18 (cat.# 9304A12) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||
| Guard Column: | Raptor C18 EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9304A0252) | ||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||
| Diluent: | Water | ||||||||||||||||||||
| Conc.: | 10–200 ng/mL | ||||||||||||||||||||
| Inj. Vol.: | 2 µL | ||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||
| A: | 0.1% Formic acid in water | ||||||||||||||||||||
| B: | 0.1% Formic acid in acetonitrile | ||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Mode: | ESI+ |
| Mode: | MRM |
| Instrument | UHPLC |
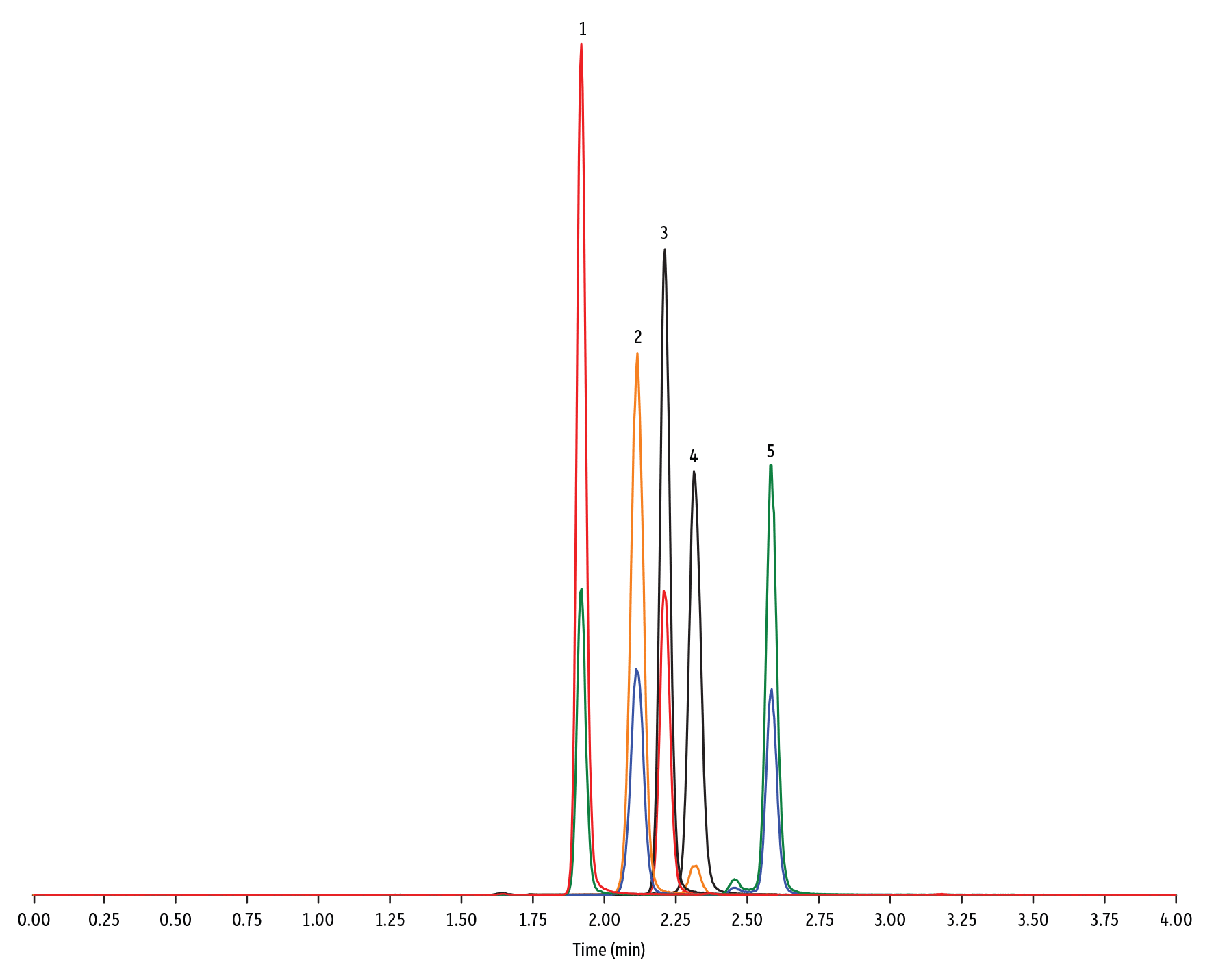
LC_FS0503
Peaks
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 1. | Lasalocid A | 1.92 | 100 | 613.42 | 377.28 | 595.40 |
| 2. | Monensin | 2.12 | 100 | 693.50 | 675.44 | 461.30 |
| 3. | Salinomycin | 2.19 | 100 | 773.57 | 431.24 | 531.39 |
| 4. | Maduramicin | 2.30 | 100 | 939.65 | 877.58 | 473.31 |
| 5. | Narasin | 2.58 | 100 | 787.59 | 431.27 | 531.35 |
Conditions
| Column | Raptor Biphenyl (cat.# 9309A12) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||
| Guard Column: | Raptor Biphenyl EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9309A0252) | ||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||
| Diluent: | Water:methanol (10:90) | ||||||||||||||||||||
| Conc.: | 100 ng/mL | ||||||||||||||||||||
| Inj. Vol.: | 2 µL | ||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||
| A: | 0.5% Formic acid in water | ||||||||||||||||||||
| B: | 0.5% Formic acid in acetonitrile | ||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Mode: | ESI+ |
| Mode: | MRM |
| Instrument | UHPLC |
| Notes | Want even better performance when analyzing metal-sensitive compounds? Check out Inert LC columns at www.restek.com/inert. |


