In a previous blog Jason Herrington mentioned a dual column MS/FID setup for China’s combined HJ759 + PAMS + HJ683 method. While this could be done with a simple Y splitter, a more elegant solution is to use a microfluidic switch, or Deans switch, to send some compounds to the secondary column and FID while maintaining the bulk of the analysis on the MS.
So how does it work? The Deans switch is composed of a pressure control module (PCM), a solenoid valve, and a 3 port switching plate. The primary column is connected to the switching plate with a short transfer line to the primary detector (MS), and a second column to the secondary detector (FID). The solenoid valve directs auxiliary carrier gas flow to the plate, with one of the outlet ports at higher pressure than the other, as shown in Fig. 1 below. The larger arrow on the MS output end of the Deans switch shows the higher pressure that directs the flow to the FID when the switch is on, and vice versa. A smaller pressure is applied to the other side to ensure that the flow from column 1 doesn’t backflow to the PCM.

Fig. 1 – Deans switch operation, with the flow from column 1 shown in red.
This has several advantages over simply splitting the flow. Since the entire sample isn’t passing through the secondary column it can be chosen without concern over it being robust enough to handle everything in the sample. No worries about trying to elute less volatile compounds off your thick film or plot columns. Also, by not splitting the sample sensitivity is maintained without having to decrease split ratios or increase injection volumes. It is important to note though that the Deans switch does increase carrier gas flow on the restrictor and column 2 due to the extra flow from the switching plate, so your MS may see a slight decrease in sensitivity. The extra flow is either 50% or at least 1mL/min more than column 1, so if your primary column flow is 2mL/min your final flow to the MS will be 3mL/min, so keep in mind the pumping efficiency of your MS.
What does it look like in the end? With no cryogenic cooling we have complete analysis of 112 VOCs in 35 minutes. The Deans switch sends the C2 and C3 hydrocarbons at the beginning of the run to the secondary column and FID for better separation and detection, then switches the rest of the run to the MS.
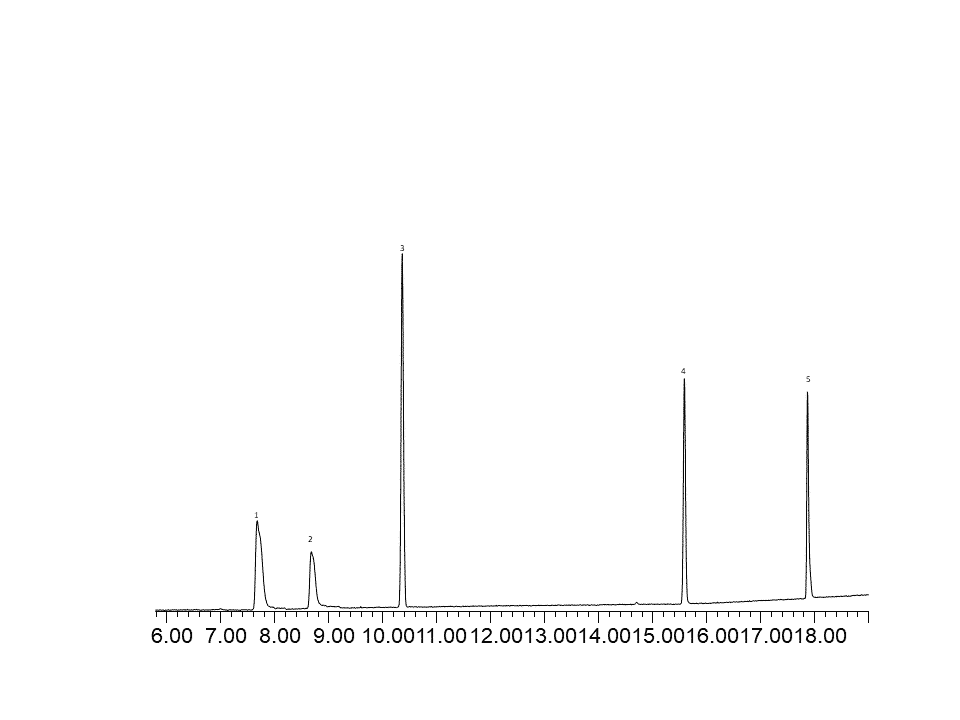
Fig.2 – FID chromatogram of C2 and C3 hydrocarbons at ~1ng on column.
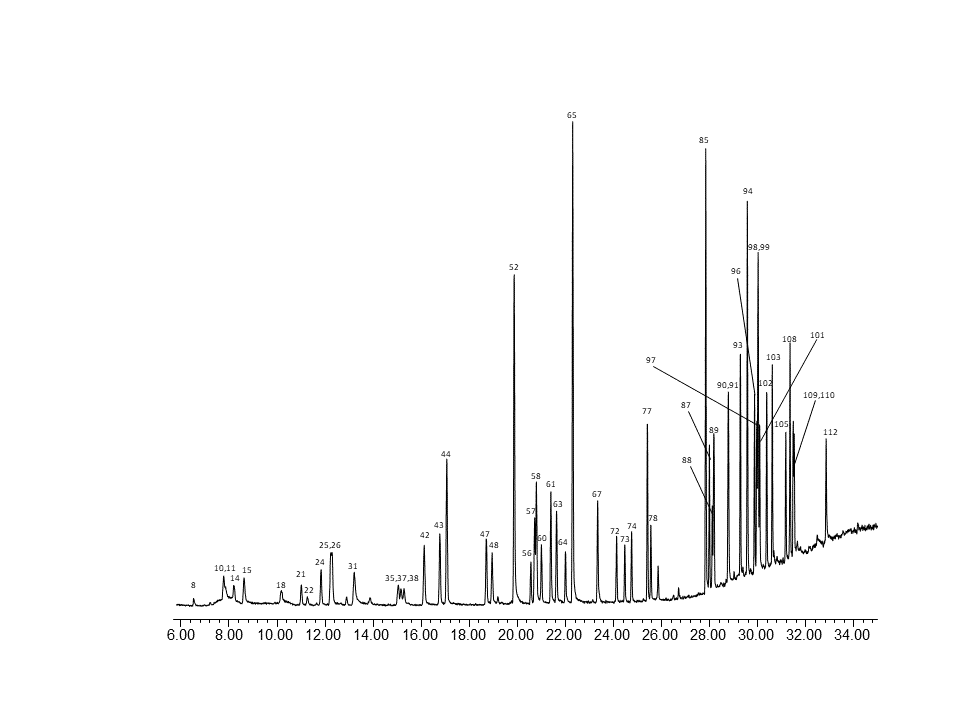
Fig. 3 – MS chromatogram of PAMS compounds at ~1ng on column.
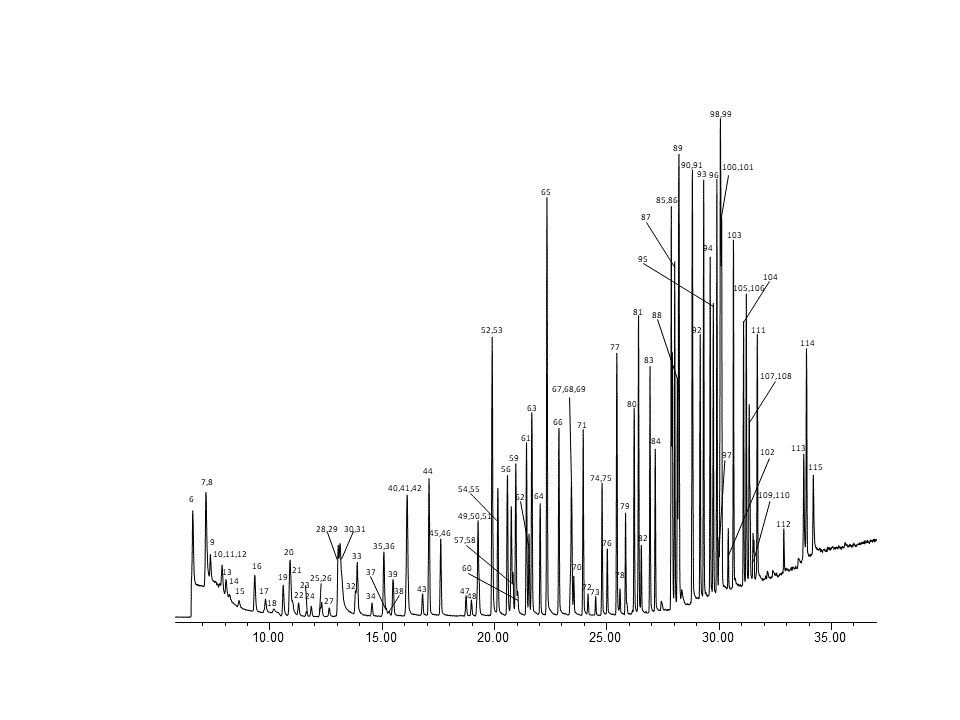
Fig. 4 –MS chromatogram of PAMS + HJ759 compounds at ~1ng on column.
| Peaks | TO-15 | PAMS | TR (min) | Peaks | TO-15 | PAMS | TR (min) | |||
| 1 | Ethane | X | 7.677 | 59 | Carbon tetrachloride | X | 20.927 | |||
| 2 | Ethylene | X | 8.68 | 60 | 3-Methylhexane | X | 21.011 | |||
| 3 | Propane | X | 10.363 | 61 | Benzene | X | X | 21.401 | ||
| 4 | Propylene | X | X | 15.583 | 62 | 1,2-Dichloroethane | X | 21.513 | ||
| 5 | Acetylene | X | 17.863 | 63 | Isooctane | X | X | 21.638 | ||
| 6 | Dichlorodifluoromethane | X | 6.567 | 64 | Heptane | X | X | 22.01 | ||
| 7 | 1,2-Dichlorotetrafluoroethane | X | 7.148 | 65 | 1,4-Difluorobenzene | X | X | 22.312 | ||
| 8 | Isobutane | X | 7.204 | 66 | Trichloroethylene | X | 22.841 | |||
| 9 | Chloromethane | X | 7.348 | 67 | Methylcyclohexane | X | 23.348 | |||
| 10 | trans-2-Butene | X | 7.761 | 68 | 1,2-Dichloropropane | X | 23.399 | |||
| 11 | n-Butane | X | X | 7.859 | 69 | Methyl methacrylate | X | 23.422 | ||
| 12 | Vinyl chloride | X | 7.868 | 70 | 1,4-Dioxane | X | 23.506 | |||
| 13 | 1,3-Butadiene | X | 8.045 | 71 | Bromodichloromethane | X | 23.915 | |||
| 14 | cis-2-butene | X | 8.193 | 72 | 2,3,4-Trimethylpentane | X | 24.128 | |||
| 15 | 1-Butene | X | 8.621 | 73 | 2-Methylheptane | X | 24.472 | |||
| 16 | Bromomethane | X | 9.322 | 74 | 3-Methylheptane | X | 24.751 | |||
| 17 | Chloroethane | X | 9.796 | 75 | cis-1,3-Dichloropropene | X | 24.755 | |||
| 18 | Isopentane | X | 10.163 | 76 | 4-Methyl-2-2pentanone (MIBK) | X | 24.988 | |||
| 19 | Vinyl bromide | X | 10.586 | 77 | Toluene | X | X | 25.415 | ||
| 20 | Trichlorofluoromethane | X | 10.888 | 78 | n-Octane | X | 25.555 | |||
| 21 | 1-Pentene | X | 11.013 | 79 | trans-1,3-Dichloropropene | X | 25.801 | |||
| 22 | n-Pentane | X | X | 11.269 | 80 | 1,1,2-Trichloroethane | X | 26.186 | ||
| 23 | Ethanol | X | 11.617 | 81 | Tetrachloroethene | X | 26.381 | |||
| 24 | trans-2-Pentene | X | 11.831 | 82 | 2-Hexanone (MBK) | X | 26.502 | |||
| 25 | Isoprene | X | 12.24 | 83 | Dibromochloromethane | X | 26.893 | |||
| 26 | cis-2-Pentene | X | 12.296 | 84 | 1,2-Dibromoethane | X | 27.125 | |||
| 27 | Acrolein | X | 12.63 | 85 | Chlorobenzene-d5 | X | X | 27.84 | ||
| 28 | 1,1-Dichloroethene | X | 13.025 | 86 | Chlorobenzene | X | 27.891 | |||
| 29 | 1,1,2-Trichlorotrifluoroethane | X | 13.113 | 87 | Ethylbenzene | X | X | 27.994 | ||
| 30 | Acetone | X | 13.192 | 88 | n-Nonane | X | X | 28.11 | ||
| 31 | 2,2-Dimethylbutane | X | 13.215 | 89 | m- & p-Xylene | X | X | 28.179 | ||
| 32 | Isopropyl alcohol | X | 13.796 | 90 | o-Xylene | X | X | 28.769 | ||
| 33 | Carbon disulfide | X | 13.875 | 91 | Styrene | X | X | 28.793 | ||
| 34 | Allyl chloride | X | 14.53 | 92 | Bromoform | X | 29.127 | |||
| 35 | 2,3-Dimethylbutane | X | 15.046 | 93 | Cumene | X | X | 29.285 | ||
| 36 | Methylene chloride | X | 15.06 | 94 | 4-Bromofluorobenzene | X | X | 29.573 | ||
| 37 | 2-Methylpentane | X | 15.176 | 95 | 1,1,2,2-Tetrachloroethane | X | 29.712 | |||
| 38 | Cyclopentane | X | 15.292 | 96 | n-Propyl benzene | X | X | 29.87 | ||
| 39 | Tertiary butanol | X | 15.469 | 97 | 1,2,3-Trimethylbenzene | X | 29.963 | |||
| 40 | Methyl tert-butyl ether (MTBE) | X | 16.068 | 98 | n-Decane | X | 30.01 | |||
| 41 | trans-1,2-Dichloroethene | X | 16.096 | 99 | p-Ethyltoluene | X | X | 30.024 | ||
| 42 | 3-Methylpentane | X | 16.133 | 100 | 2-Chlorotoluene | X | 30.052 | |||
| 43 | 1-Hexene | X | 16.783 | 101 | 1,3,5-Trimethylbenzene | X | X | 30.084 | ||
| 44 | Hexane | X | X | 17.071 | 102 | m-Ethyltoluene | X | 30.377 | ||
| 45 | 1,1-Dichloroethane | X | 17.587 | 103 | 1,2,4-Trimethylbenzene | X | X | 30.609 | ||
| 46 | Vinyl acetate | X | 17.587 | 104 | 1,3-Dichlorobenzene | X | 31.06 | |||
| 47 | 2,4-Dimethylpentane | X | 18.711 | 105 | o-Ethyltoluene | X | 31.171 | |||
| 48 | Methylcyclopentane | X | 18.958 | 106 | 1,4-Dichlorobenzene | X | 31.185 | |||
| 49 | 2-Butanone (MEK) | X | 19.194 | 107 | Benzyl chloride | X | 31.311 | |||
| 50 | cis-1,2-Dichloroethene | X | 19.246 | 108 | m-Diethylbenzene | X | 31.352 | |||
| 51 | Ethyl acetate | X | 19.297 | 109 | p-Diethylbenzene | X | 31.483 | |||
| 52 | Bromochloromethane | X | X | 19.873 | 110 | n-Undecane, | X | 31.524 | ||
| 53 | Tetrahydrofuran | X | 19.896 | 111 | 1,2-Dichlorobenzene | X | 31.673 | |||
| 54 | Chloroform | X | 20.128 | 112 | n-Dodecane | X | 32.853 | |||
| 55 | 1,1,1-Trichloroethane | X | 20.551 | 113 | 1,2,4-Trichlorobenzene | X | 33.745 | |||
| 56 | 2-methylhexane | X | 20.57 | 114 | Hexachlorobutadiene | X | 33.866 | |||
| 57 | Cyclohexane | X | X | 20.723 | 115 | Naphthalene | X | 34.172 | ||
| 58 | 2,3-Dimethylpentane | X | 20.797 |
That covers the PAMS and HJ759 methods, but what about HJ683? Don’t worry, there’s more to come on this application soon.



