Key Highlights
- Simple, streamlined sample preparation protocol minimizes contamination and maximizes recovery.
- Inert column hardware packed with a unique polar-embedded stationary phase and established method conditions provide accurate, precise results for the simultaneous analysis of ultrashort-chain, short-chain, medium-chain, long-chain, and alternative PFAS.
- Proven suitable for comprehensive assessment of PFAS contamination across a diverse range of liquid milk samples.
Abstract
Incorporating ultrashort-chain PFAS into methods that also include short-chain, medium-chain, long-chain and alternative PFAS is essential for gaining a comprehensive assessment of PFAS contamination. The sample preparation and LC-MS/MS workflow established here provides an effective approach for quantitative monitoring of a diverse panel of PFAS, including ultrashort-chain analytes, in a wide range of milk matrices. The method was assessed based on linearity, accuracy, and precision parameters and then applied to real-world samples of various dairy milks; plant-based milks; and infant formula.
Introduction
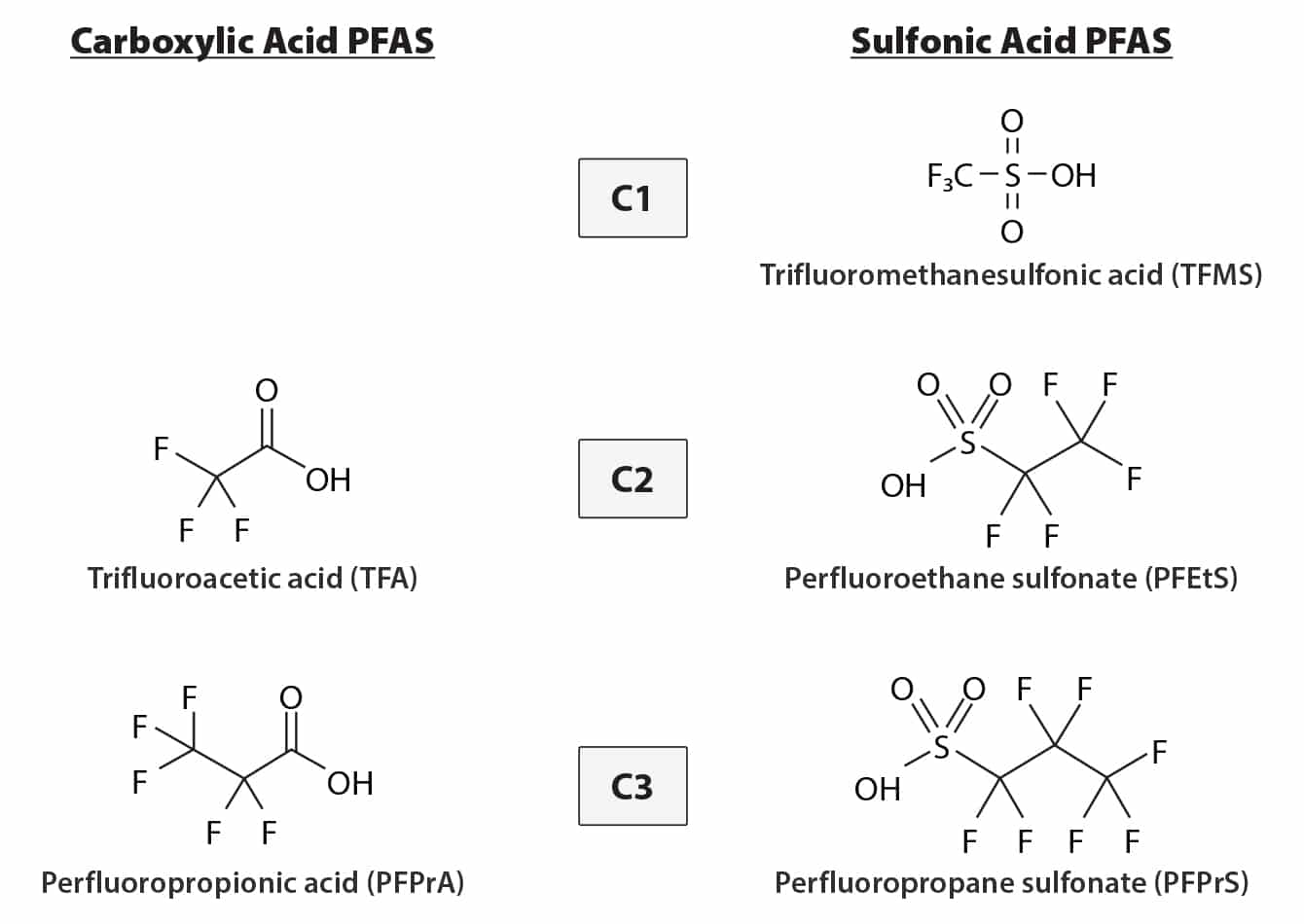
Ultrashort-chain (USC) per- and polyfluoroalkyl substances (PFAS) are highly polar compounds with carbon chains shorter than C4 (Figure 1), and they are ubiquitous in aquatic environments. Their widespread occurrence has raised growing concerns about potential contamination in food products, particularly in ready-to-feed liquid milk products, which are widely consumed by infants and children. While effective methods exist for analyzing longer-chain PFAS in milk samples [1], to fully assess PFAS contamination, it is critical to include ultrashort-chain compounds.
Analyzing PFAS in milk poses unique challenges due to the complexity of the matrix, which contains proteins, fats, and other components that can interfere with detection. Ultrashort-chain PFAS and other shorter-chain compounds are particularly difficult to analyze, as traditional multi-step sample preparation methods can either contribute background PFAS contamination or lead to low recovery of the target analytes. In this study, a sample preparation procedure involving protein precipitation, extract dry-down, and reconstitution was developed and optimized for effective extraction and quantification of all analytes. In addition to sample preparation challenges, the high polarity of ultrashort-chain PFAS poses a significant difficulty for standard chromatographic practices in PFAS analysis, primarily due to insufficient chromatographic retention. The method developed here uses an inert-coated, polar-embedded, reversed-phase LC column to improve retention and sensitivity.
The combined sample preparation procedure and chromatographic method created a simple, reliable workflow for the simultaneous analysis of C1 to C14 perfluoroalkyl carboxylic and sulfonic acids, along with other PFAS classes, in various liquid milk matrices. Method verification was conducted using three different milk types (dairy milk; plant-based milk; and infant formula) to establish the workflow’s suitability for detecting 41 PFAS. In addition, the method was tested across a wide range of real-world milk samples, demonstrating its effectiveness in comprehensive profiling of PFAS contamination across diverse milk matrices.
Figure 1: Structures of Ultrashort-Chain PFAS (C1 to C3)
Experimental
Evaluation of Background Contamination of Ultrashort-Chain PFAS
Given the widespread presence of ultrashort-chain PFAS in aquatic systems, ensuring the cleanliness of laboratory reagents and materials is crucial for accurate analysis. In particular, background contamination of TFA is a major concern, as it can be present in solvents, reagents, and commonly used laboratory materials. Through extensive testing, the cleanest sources of water, acetonitrile, and methanol were identified for this study. Notably, significant variations in TFA detection signals were observed with different water sources used for the aqueous mobile phase, leading to the selection of ultrapure deionized water to ensure optimal sensitivity. Likewise, various pipette tips, HPLC vials, and centrifuge tubes used for standard and sample preparation were evaluated for contamination, allowing selection of the cleanest materials to maintain the integrity of our results.
Standard and Sample Preparation
Calibration standard solutions (500 µL) were prepared in polypropylene HPLC vials using a 1:1 mixture of reverse osmosis water and acetonitrile, with concentrations ranging from 4 to 2500 ng/L. Eighteen mass-labeled PFAS were used as quantitative internal standards (QIS) (Table I). A 2.5 µL aliquot of QIS working solution, containing 10 ng/mL of each isotope, was added to each standard solution. The milk samples (0.5 g) were weighed into 15-mL polypropylene centrifuge tubes, spiked with 2.5 µL of 10 ng/mL QIS working solution, thoroughly mixed, and extracted with 1.5 mL of acetonitrile by vortexing for 2 minutes. The mixture was then centrifuged at 4000 rpm, and then the supernatant was transferred to a new 15-mL tube and dried under a gentle nitrogen stream in a 50 °C water bath. The dried residue was reconstituted with 0.5 mL of a 1:1 water:acetonitrile diluent, vortexed for 2 minutes, and centrifuged again at 4000 rpm. The final supernatant was transferred to polypropylene HPLC vials for LC-MS/MS analysis.
Evaluation of Method Accuracy and Precision
To demonstrate that the developed workflow can be applied to various milk matrices, three types of ready-to-feed milk samples—dairy whole milk, almond milk, and infant formula—were used to evaluate method accuracy and precision. Almond milk was selected to represent the plant-based milk due to its widespread consumption. These milk samples were fortified with native analytes at concentrations of 0.010, 0.025, 0.050, 0.10, and 0.25 µg/kg, along with isotopically labeled 13C-TFA, which served as a surrogate for TFA recovery determination. These concentrations correspond to 10, 25, 50, 100, and 250 ng/L in the final sample solution following the sample preparation procedure described above. Incurred PFAS were detected in all three milk samples, and their concentrations were subtracted from the calculated concentrations of fortified samples to determine the recovery. Table I illustrates the pairing of QIS with various analytes for quantification. Due to differential matrix effects, some analytes in almond milk required alternative QIS for more accurate quantification.
Analytical Conditions
In this approach for incorporating ultrashort-chain PFAS and related compounds into a single method, analysis was performed by LC-MS/MS using a Waters ACQUITY UPLC I-Class liquid chromatograph and Xevo TQ-S triple quadrupole mass spectrometer under the conditions shown below. Ion transitions, MS settings, and the internal standard used to quantify each PFAS are given in Table I. An Ultra IBD column (150 × 2.1 mm, 3.0 µm) was employed as the delay column to more effectively remove background contamination originating from the instrument and mobile phases. Although a standard C18 PFAS delay column is typically adequate, it did not provide acceptable separation between background contaminants and target analytes when coupled with the Ultra Inert IBD analytical column.
| Columns: – Analytical column: Ultra Inert IBD, 3.0 µm, 100 x 2.1 mm (cat.# 9175312-T) – Delay column: Ultra IBD, 3.0 µm, 150 x 2.1 mm (cat.# 9175362) Injection volume: 5 µL Mobile phase A: 5 mM ammonium formate, 0.1% formic acid in water Mobile phase B: Acetonitrile Flow rate: 0.4 mL/min Temperature: 40 °C Gradient: Time (min) %B 0.00 45 7.00 95 11.00 95 11.01 45 13.00 45 Ion mode: Negative ESI Mode: Scheduled MRM |
Table I: MS/MS Parameters and Internal Standards
| Compounds | Retention Time (min) | Precursor Ion | Product Ionsa | Cone (V) | Collision (V) | QIS |
|---|---|---|---|---|---|---|
| Target Analytes | ||||||
| Perfluoroalkyl Carboxylic Acids | ||||||
| Trifluoroacetic acid (TFA) | 1.60 | 113.03 [M-H]- | 69.01 | 10 | 10 | 13C2-TFA |
| Perfluoropropanoic acid (PFPrA) | 2.21 | 162.97 [M-H]- | 119.02 | 10 | 8 | 13C3-PFPrA |
| Perfluorobutanoic acid (PFBA) | 2.86 | 213.03 [M-H]- | 168.98 | 14 | 8 | 13C4-PFBA |
| Perfluoropentanoic acid (PFPeA) | 3.64 | 262.97 [M-H]- | 218.97 | 2 | 6 | 13C5-PFPeA |
| Perfluorohexanoic acid (PFHxA) | 4.41 | 313.10 [M-H]- | 268.97/118.99 | 2 | 8/20 | 13C5-PFHxA |
| Perfluoroheptanoic acid (PFHpA) | 5.15 | 363.16 [M-H]- | 319.09/169.06 | 8 | 10/18 | 13C4-PFHpA |
| Perfluorooctanoic acid (PFOA) | 5.84 | 413.10 [M-H]- | 368.96/168.90 | 2 | 10/16 | 13C8-PFOA |
| Perfluorononanoic acid (PFNA) | 6.48 | 463.10 [M-H]- | 419.01/219.02 | 4 | 10/16 | 13C9PFNA |
| Perfluorodecanoic acid (PFDA) | 7.08 | 513.17 [M-H]- | 469.16/219.06 | 4 | 12/16 | 13C6-PFDA |
| Perfluoroundecanoic acid (PFUnA) | 7.65 | 563.23 [M-H]- | 519.24/269.07 | 6 | 12/18 | 13C7-PFUnA |
| Perfluorododecanoic acid (PFDoA) | 8.26 | 613.23 [M-H]- | 569.19/169.06 | 8 | 12/26 | 13C2-PFDoA |
| Perfluorotridecanoic acid (PFTrDA) | 8.94 | 663.23 [M-H]- | 619.21/169.06 | 8 | 14/28 | 13C2-PFTeDA |
| Perfluorotetradecanoic acid (PFTeDA) | 9.75 | 712.67 [M-H]- | 668.69/168.94 | 10 | 12/26 | 13C2-PFTeDA |
| Perfluoroalkyl Sulfonic Acids | ||||||
| Trifluoromethanesulfonic acid (TFMS) | 1.98 | 148.97 [M-H]- | 79.93/98.92 | 62 | 18/18 | 13C3-PFBS |
| Perfluoroethanesulfonic acid (PFEtS) | 2.62 | 198.90 [M-H]- | 79.92/98.91 | 38 | 22/22 | 13C4-PFBA |
| Perfluoropropanesulfonic acid (PFPrS) | 3.30 | 248.97 [M-H]- | 79.92/98.91 | 2 | 24/24 | 13C5-PFPeA |
| Perfluorobutanesulfonic acid (PFBS) | 3.96 | 298.97 [M-H]- | 79.97/98.89 | 2 | 26/26 | 13C3-PFBS |
| Perfluoropentanesulfonic acid (PFPeS) | 4.59 | 349.10 [M-H]- | 79.98/98.98 | 6 | 32/30 | 13C5-PFHxA / 13C8-PFOSb |
| Perfluorohexanesulfonic acid (PFHxS) | 5.17 | 398.90 [M-H]- | 79.97/98.89 | 56 | 32/34 | 13C3-PFHxS |
| Perfluoroheptanesulfonic acid (PFHpS) | 5.70 | 449.17 [M-H]- | 79.98/98.97 | 4 | 42/38 | 13C8-PFOA |
| Perfluorooctanesulfonic acid (PFOS) | 6.19 | 499.03 [M-H]- | 79.92/98.90 | 8 | 40/40 | 13C8-PFOS |
| Perfluorononanesulfonic acid (PFNS) | 6.65 | 549.10 [M-H]- | 79.92/98.83 | 12 | 42/40 | 13C8-PFOS |
| Perfluorodecanesulfonic acid (PFDS) | 7.06 | 599.17 [M-H]- | 79.98/98.83 | 8 | 44/46 | 13C8-PFOS / 13C2-PFDoAb |
| Perfluoroundecanesulfonic acid (PFUdS) | 7.43 | 648.73 [M-H]- | 79.94/98.94 | 38 | 50/44 | 13C8-PFOS / 13C2-PFDoAb |
| Perfluorododecanesulfonic acid (PFDoS) | 7.77 | 698.77 [M-H]- | 79.95/98.94 | 10 | 60/44 | 13C8-PFOS / 13C2-PFDoAb |
| Perfluorotridecanesulfonic acid (PFTrDS) | 8.08 | 748.73 [M-H]- | 79.94/98.94 | 8 | 76/52 | 13C8-PFOS / 13C2-PFDoAb |
| Fluorotelomer Sulfonic Acids | ||||||
| 1H,1H,2H,2H-Perfluorohexane sulfonic acid (4:2 FTS) | 3.92 | 327.10 [M-H]- | 307.08/80.83 | 50 | 18/24 | 13C4-PFBA / 13C5-PFHxAb |
| 1H,1H,2H,2H-Perfluorooctane sulfonic acid (6:2 FTS) | 5.57 | 427.17 [M-H]- | 407.18/80.71 | 2 | 22/32 | 13C8-PFOA |
| 1H,1H,2H,2H-Perfluorodecane sulfonic acid (8:2 FTS) | 7.07 | 527.17 [M-H]- | 507.16/80.83 | 66 | 26/32 | 13C3-PFHxS / d5-NEtFOSAAb |
| Perfluoroalkyl Sulfonamides | ||||||
| Perfluorooctanesulfonamide (FOSA) | 4.01 | 498.17 [M-H]- | 77.97/477.76 | 8 | 28/26 | 13C8-FOSA |
| Perfluoroalkyl Sulfonamidoacetic Acids | ||||||
| N-methyl perfluorooctanesulfonamidoacetic acid (NMeFOSAA) | 6.40 | 570.20 [M-H]- | 419.17/483.16 | 46 | 20/14 | d3-NMeFOSAA |
| N-ethyl perfluorooctanesulfonamidoacetic acid (NEtFOSAA) | 6.52 | 584.20 [M-H]- | 419.18/483.11 | 6 | 20/16 | d5-NEtFOSAA |
| Per- and Polyfluoroether Carboxylic Acids | ||||||
| Perfluoro-3-methoxypropanoic acid (PFMPA) | 3.05 | 228.93 [M-H]- | 84.97/198.94 | 10 | 10/14 | 13C4-PFBA |
| Perfluoro-4-methoxybutanoic acid (PFMBA) | 3.76 | 278.87 [M-H]- | 84.96/234.93 | 8 | 10/6 | 13C5-PFPeA |
| Hexafluoropropylene oxide dimer acid (HFPO-DA) | 4.37 | 285.03 [M-COOH]- | 169.02/185.02 | 2 | 6/16 | 13C5-PFHxA |
| 4,8-Dioxa-3H-perfluorononanoic acid (ADONA) | 4.71 | 376.90 [M-H]- | 250.93/84.97 | 22 | 12/26 | 13C4-PFHpA |
| Per- and Polyfluoroether Sulfonic Acids | ||||||
| Perfluoro(2-ethoxyethane)sulfonic acid (PFEESA) | 4.09 | 314.83 [M-H]- | 134.94/83.01 | 4 | 22/16 | 13C3-PFBS |
| 9-Chlorohexadecafluoro-3-oxanonane-1-sulfonic acid (9Cl-PF3ONS) | 6.20 | 530.78 [M-H]- | 350.85/82.96 | 12 | 26/24 | 13C8-PFOA |
| 11-Chloroeicosafluoro-3-oxaundecane-1-sulfonic acid (11Cl-PF3OUdS) | 6.92 | 630.78 [M-H]- | 450.80/82.95 | 8 | 26/32 | 13C8-PFOS |
| Capstone Surfactants | ||||||
| Capstone A | 0.95 | 527.08 [M-H]- | 507.02/181.06 | 2 | 10/12 | 13C2-TFA / 13C3-PFPrAb |
| Capstone B | 1.90 | 569.07 [M-H]- | 549.01/445.96 | 32 | 12/16 | 13C3-PFPrA |
| Quantification Internal Standards | ||||||
| 13C2-TFA | 2.69 | 114.90 [M-H]- | 69.95 | 14 | 8 | |
| 13C3-PFPrA | 2.69 | 165.97 [M-H]- | 120.96 | 10 | 11 | |
| 13C4-PFBA | 3.27 | 217.03 [M-H]- | 171.98 | 2 | 8 | |
| 13C5-PFPeA | 3.94 | 267.97 [M-H]- | 222.99 | 2 | 6 | |
| 13C5-PFHxA | 4.59 | 318.03 [M-H]- | 272.93 | 2 | 7 | |
| 13C4-PFHpA | 5.24 | 366.90 [M-H]- | 321.93 | 2 | 10 | |
| 13C8-PFOA | 5.86 | 420.97 [M-H]- | 375.94 | 2 | 10 | |
| 13C9PFNA | 5.86 | 471.97 [M-H]- | 426.87 | 4 | 12 | |
| 13C6-PFDA | 7.03 | 518.90 [M-H]- | 473.87 | 4 | 13 | |
| 13C7-PFUnA | 7.60 | 569.90 [M-H]- | 524.87 | 2 | 12 | |
| 13C2-PFDoA | 8.23 | 614.84 [M-H]- | 569.87 | 2 | 12 | |
| 13C2-PFTeDA | 9.83 | 714.78 [M-H]- | 669.80 | 8 | 14 | |
| 13C3-PFBS | 3.97 | 301.97 [M-H]- | 79.97 | 2 | 28 | |
| 13C3-PFHxS | 5.01 | 401.90 [M-H]- | 79.97 | 2 | 36 | |
| 13C8-PFOS | 5.96 | 506.84 [M-H]- | 79.97 | 4 | 42 | |
| 13C8-FOSA | 3.36 | 505.91 [M-H]- | 77.95 | 4 | 32 | |
| d3-NMeFOSAA | 6.44 | 572.90 [M-H]- | 418.91 | 50 | 18 | |
| d5-NEtFOSAA | 6.56 | 588.97 [M-H]- | 418.86 | 48 | 20 | |
| aQuantifier ion/qualifier ion bUsed for quantification in almond milk | ||||||
Results and Discussion
Chromatographic Performance
Analytical methods were previously developed for incorporating ultrashort-chain PFAS into comprehensive methods for testing PFAS in various water matrices [2] and human blood [3]. These methods utilized a polar-embedded alkyl Ultra IBD stationary phase to ensure adequate retention of highly polar ultrashort-chain PFAS. Additionally, it was demonstrated that an inert-coated Ultra IBD column could significantly enhance detection sensitivity for the majority of PFAS compounds [2] because it is made with hardware treated with an inert coating that prevents any unwanted analyte interactions with the stainless-steel surface of the column. The current study also applied a similar chromatographic methodology for PFAS analysis in milk samples. However, due to stronger matrix interference compared to water matrices, the injection volume was limited to 5 µL to maintain optimal chromatographic peak shapes for early-eluting compounds.
Figure 2 shows a typical chromatogram for the analysis of a fortified milk sample. In addition to the 28 PFAS compounds assessed under EURL guidance [4], this analysis was expanded to 41 analytes by incorporating ultrashort-chain PFAS, fluorotelomer sulfonic acids, and a broader range of alternative PFAS compounds. The EURL guidance notes that bile acids, such as tauroursodeoxycholic acid (TUDCA); taurochenodeoxycholic acid (TCDCA); and taurodeoxycholic acid (TDCA) can be present at high concentrations in milk samples and may interfere with PFOS quantification due to their shared mass transitions. Our tests confirmed that TUDCA, TCDCA, and TDCA all had distinct retention times from PFOS, ensuring they will not influence the accuracy of PFOS results (Figure 3). Furthermore, these bile acids displayed significantly lower detection sensitivity (~100-fold lower) than PFOS and were not detected in the final milk sample solutions analyzed in this study.
Figure 2: Analysis of a 0.1 µg/kg Fortified Whole Milk Sample
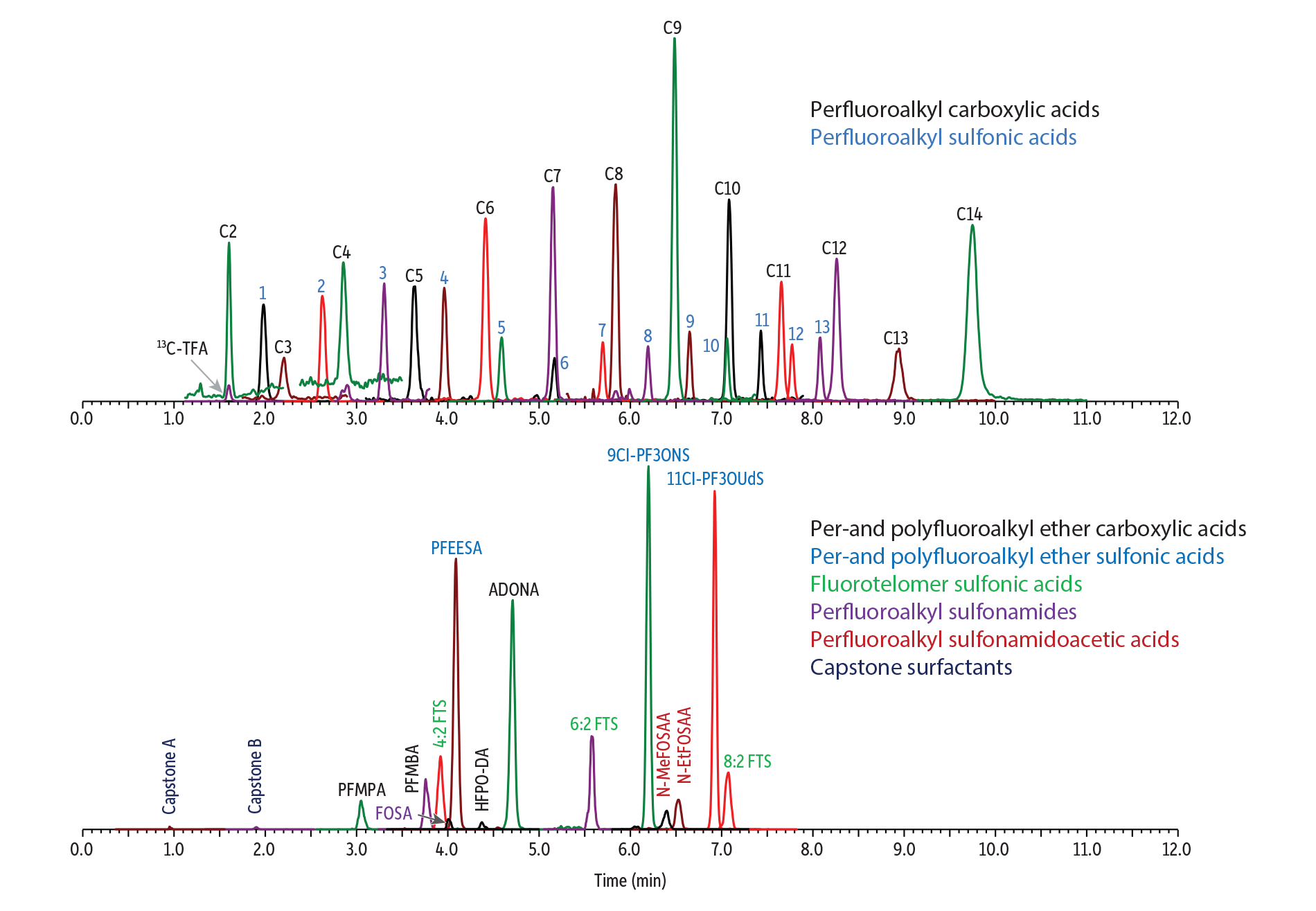
LC_FS0563
Peaks
| Peaks | tR (min) | Precursor Ion | Quantification Ion | Confirmation Ion | |
|---|---|---|---|---|---|
| 1. | Capstone A | 0.95 | 527.08 | 507.02 | 181.06 |
| 2. | Trifluoroacetic acid (TFA) | 1.60 | 113.03 | 69.01 | |
| 3. | 13C-Trifluoroacetic acid (13C-TFA) | 1.60 | 114.03 | 69.03 | |
| 4. | Capstone B | 1.90 | 569.07 | 549.01 | 445.96 |
| 5. | Trifluoromethanesulfonic acid (TFMS) | 1.98 | 148.97 | 79.93 | 98.92 |
| 6. | Perfluoropropanoic acid (PFPrA) | 2.21 | 162.97 | 119.02 | |
| 7. | Perfluoroethanesulfonic acid (PFEtS) | 2.62 | 198.90 | 79.92 | 98.91 |
| 8. | Perfluoro-3-methoxypropanoic acid (PFMPA) | 3.05 | 228.93 | 84.97 | 198.94 |
| 9. | Perfluoropropanesulfonic acid (PFPrS) | 3.30 | 248.97 | 79.92 | 98.91 |
| 10. | Perfluoropentanoic acid (PFPeA) | 3.64 | 262.97 | 218.97 | |
| 11. | Perfluoro-4-methoxybutanoic acid (PFMBA) | 3.76 | 278.87 | 84.96 | 234.93 |
| 12. | 1H,1H,2H,2H-Perfluorohexane sulfonic acid (4:2 FTS) | 3.92 | 327.10 | 307.08 | 80.83 |
| 13. | Perfluorobutanesulfonic acid (PFBS) | 3.96 | 298.97 | 79.97 | 98.89 |
| 14. | Perfluorooctanesulfonamide (FOSA) | 4.01 | 498.17 | 77.97 | 477.76 |
| 15. | Perfluoro(2-ethoxyethane)sulfonic acid (PFEESA) | 4.09 | 314.83 | 134.94 | 83.01 |
| 16. | Hexafluoropropylene oxide dimer acid (HFPO-DA) | 4.37 | 285.03 | 169.02 | 185.02 |
| 17. | Perfluorohexanoic acid (PFHxA) | 4.41 | 313.10 | 268.97 | 118.99 |
| 18. | Perfluoropentanesulfonic acid (PFPeS) | 4.59 | 349.10 | 79.98 | 98.98 |
| 19. | 4,8-Dioxa-3H-perfluorononanoic acid (ADONA) | 4.71 | 376.90 | 250.93 | 84.97 |
| 20. | Perfluoroheptanoic acid (PFHpA) | 5.15 | 363.16 | 319.09 | 169.06 |
Conditions
| Column | Ultra Inert IBD (cat.# 9175312-T) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||||||
| Particle Size: | 3 µm | ||||||||||||||||||||||||
| Pore Size: | 100 Å | ||||||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||
| PFAS 28 calibration standard (cat.# 30734) | |||||||||||||||||||||||||
| Other standards were obtained externally | |||||||||||||||||||||||||
| Other standards were obtained externally | |||||||||||||||||||||||||
| Diluent: | 50:50 water:acetonitrile | ||||||||||||||||||||||||
| Conc.: | Whole milk fortified at 0.1 μg/kg | ||||||||||||||||||||||||
| Inj. Vol.: | 5 µL | ||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||
| A: | Water, 5mM ammonium formate, 0.1% formic acid | ||||||||||||||||||||||||
| B: | Acetonitrile | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Max Pressure: | 540 bar |
| Detector | Waters Xevo TQ-S |
|---|---|
| Ion Source: | Waters Zspray ESI |
| Ion Mode: | ESI- |
| Mode: | MRM |
| Instrument | Waters ACQUITY UPLC I-Class |
| Sample Preparation | Milk samples (0.5 g) were weighed into 15 mL polypropylene centrifuge tubes, spiked with analytes at 0.1 μg/kg, thoroughly mixed, and extracted with 1.5 mL of acetonitrile by vortexing for 2 minutes. The mixture was then centrifuged at 4000 rpm, and the supernatant was transferred to a new 15 mL tube and dried under a gentle nitrogen stream in a 50°C water bath. The dried residue was reconstituted with 0.5 mL of a 1:1 water:acetonitrile diluent, vortexed for 2 minutes, and centrifuged again at 4000 rpm. The final supernatant was transferred to polypropylene HPLC vials for LC-MS/MS analysis. |
| Notes | The confirmation ion peaks were not shown in the chromatogram. An Ultra IBD column (150 x 2.1 mm, 3.0 μm; cat # 9175362) was used as the delay column. For the chromatograms of perfluoroalkyl carboxylic and sulfonic acids, the peak numbers correspond to the carbon chain lengths of the PFAS compounds. |
Figure 3: Full Chromatographic Separation of PFOS and Potentially Interfering Bile Acids
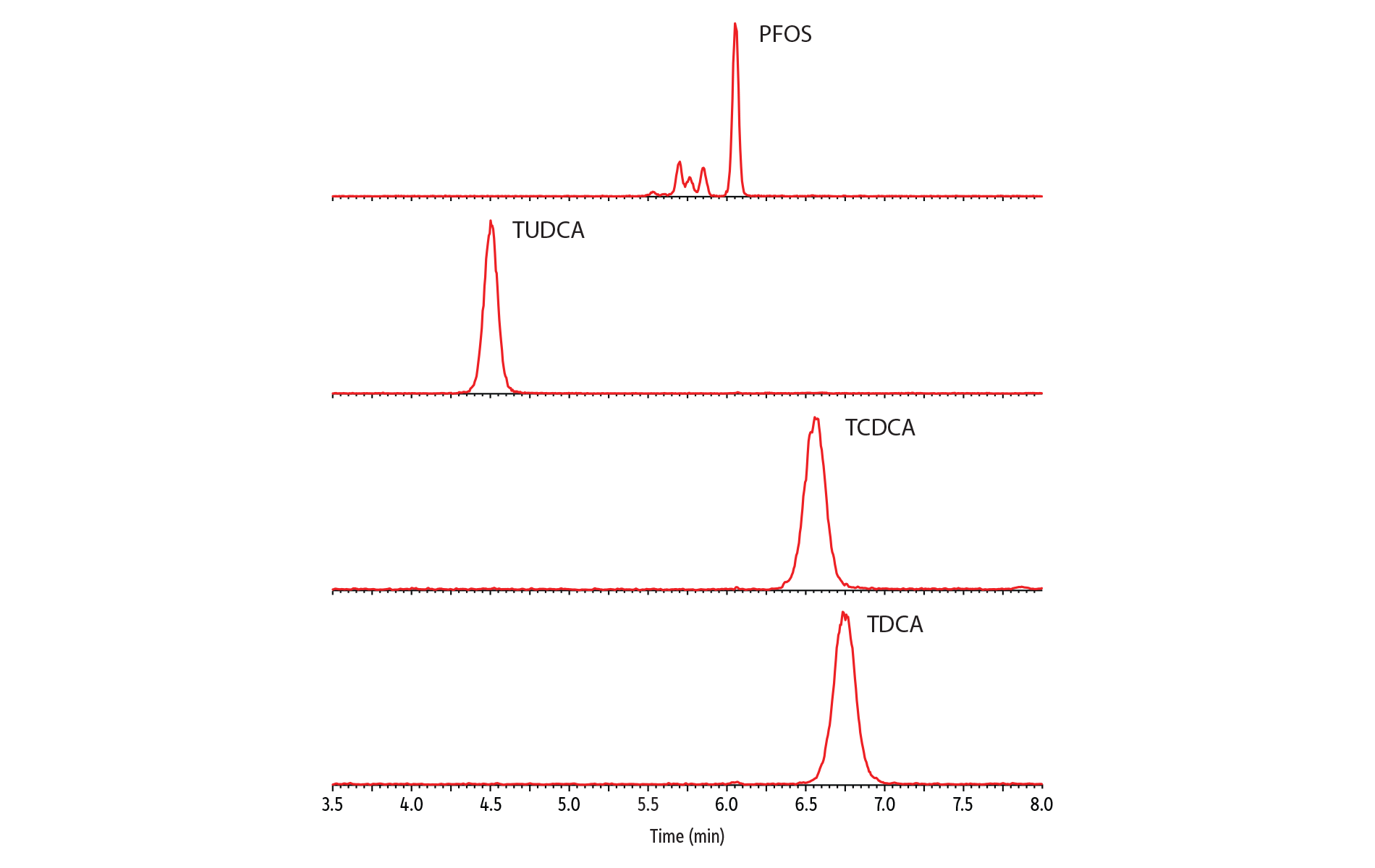
LC_FS0565
Peaks
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Confirmation Ion | |
|---|---|---|---|---|---|
| 1. | Tauroursodeoxycholic acid (TUDCA) | 4.50 | 100 | 499.03 | 79.92 |
| 2. | Perfluorooctanesulfonic acid (PFOS) | 6.05 | 0.5 | 499.03 | 79.92 |
| 3. | Taurochenodeoxycholic acid (TCDCA) | 6.55 | 100 | 499.03 | 79.92 |
| 4. | Taurodeoxycholic acid (TDCA) | 6.74 | 100 | 499.03 | 79.92 |
Conditions
| Column | Ultra Inert IBD (cat.# 9175312-T) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||||||
| Particle Size: | 3 µm | ||||||||||||||||||||||||
| Pore Size: | 100 Å | ||||||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||||||
| Standard/Sample | Individual standards were obtained externally. | ||||||||||||||||||||||||
| Diluent: | 50:50 water:acetonitrile | ||||||||||||||||||||||||
| Inj. Vol.: | 5 µL | ||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||
| A: | Water, 5 mM ammonium formate, 0.1% formic acid | ||||||||||||||||||||||||
| B: | Acetonitrile | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Max Pressure: | 540 bar |
| Detector | Waters Xevo TQ-S |
|---|---|
| Ion Source: | Waters Zspray ESI |
| Ion Mode: | ESI- |
| Mode: | MRM |
| Instrument | Waters ACQUITY UPLC I-Class |
| Sample Preparation | Individual standards were prepared in 50:50 water:acetonitrile solution in polypropylene HPLC vials. |
| Notes | An Ultra IBD column (150 x 2.1 mm, 3.0 μm; cat # 9175362) was used as the delay column. |
Linearity
Employing quadratic regression (1/x weighted), all analytes exhibited acceptable linearities with r2 >0.995 and deviations <30%. The calibration ranges were 20–2500 ng/L for TFA; 20–1000 ng/L for HFPO-DA, capstone A, and capstone B; and 4–1000 ng/L for the remaining analytes.
Accuracy and Precision
Three batches of analyses were conducted on different days, totaling nine replicates at each fortified level. The average recovery and relative standard deviation (RSD) for each PFAS in each matrix are presented in Table III. All analytes exhibited recovery values within the range of 78.3–119% in whole milk; 81.6–129% in almond milk; and 80.5–118% in infant formula. Satisfactory method precision was demonstrated by the %RSD values being ≤15%. Due to higher matrix suppression or poor detection signals, the recovery of 13C-TFA; PFPrA; FOSA; HFPO-DA; capstone A; capstone B; and PFMPA could not be determined in some or all of the 0.01 µg/kg fortified samples. Capstone A could not be identified and measured in almond milk due to specific matrix suppression.
Table III: Accuracy and Precision Analysis of PFAS in Fortified Milk Samples
Whole Milk
| Average Recovery (RSD, %), n=9 | |||||
|---|---|---|---|---|---|
| Fortified Concentration (µg/kg) | |||||
| Analytes | 0.010 | 0.025 | 0.050 | 0.10 | 0.25 |
| 13C-TFA | – | 104 (9.40) | 108 (6.19) | 111 (7.65) | 114 (6.02) |
| PFPrA | 110 (9.22) | 108 (9.29) | 114 (5.59) | 112 (7.55) | 119 (4.73) |
| PFBA | 115 (3.43) | 104 (6.80) | 111 (8.73) | 108 (4.26) | 112 (3.60) |
| PFPeA | 106 (8.48) | 91.3 (8.68) | 92.0 (10.0) | 97.5 (12.4) | 102 (6.73) |
| PFHxA | 108 (4.27) | 87.9 (7.77) | 92.6 (9.97) | 90.9 (10.8) | 105 (5.71) |
| PFHpA | 113 (4.65) | 93.7 (6.82) | 92.1 (8.58) | 90.3 (6.51) | 103 (9.38) |
| PFOA | 112 (9.69) | 91.6 (12.9) | 83.3 (11.4) | 86.9 (4.64) | 103 (10.5) |
| PFNA | 114 (7.32) | 102 (7.47) | 101 (6.33) | 105 (5.50) | 110 (4.46) |
| PFDA | 110 (10.7) | 86.4 (8.71) | 88.8 (8.37) | 95.3 (10.8) | 103 (9.83) |
| PFUnA | 109 (8.75) | 87.3 (11.8) | 95.1 (9.90) | 95.0 (10.7) | 96.4 (13.9) |
| PFDoA | 113 (7.22) | 81.7 (8.30) | 88.5 (9.97) | 93.6 (6.06) | 97.5 (15.0) |
| PFTrDA | 113 (8.61) | 85.4 (4.62) | 86.7 (4.92) | 95.2 (9.83) | 95.4 (3.12) |
| PFTeDA | 105 (13.5) | 78.9 (7.25) | 85.1 (12.2) | 96.1 (8.25) | 98.3 (15.1) |
| TFMS | 112 (7.81) | 107 (10.4) | 112 (5.96) | 82.9 (5.82) | 84.6 (7.92) |
| PFEtS | 104 (9.39) | 103 (7.51) | 105 (7.90) | 114 (5.96) | 116 (5.55) |
| PFPrS | 95.7 (5.72) | 87.6 (7.53) | 94.5 (5.08) | 91.3 (12.9) | 99.7 (7.59) |
| PFBS | 104 (12.7) | 95.8 (13.0) | 99.1 (9.98) | 100 (10.3) | 109 (8.59) |
| PFPeS | 102 (9.04) | 93.5 (12.4) | 90.2 (11.5) | 93.8 (14.0) | 107 (13.5) |
| PFHxS | 104 (8.83) | 90.6 (12.4) | 85.2 (8.26) | 88.2 (8.90) | 108 (10.4) |
| PFHpS | 85.9 (4.54) | 85.0 (4.97) | 84.6 (8.85) | 83.5 (3.94) | 97.4 (10.0) |
| PFOS | 109 (6.76) | 102 (10.3) | 102 (7.83) | 93.0 (13.7) | 102 (10.5) |
| PFNS | 104 (10.8) | 88.4 (11.0) | 96.8 (14.1) | 86.9 (5.59) | 103 (10.4) |
| PFDS | 98.5 (12.7) | 91.7 (12.0) | 97.3 (13.5) | 95.8 (14.3) | 106 (7.60) |
| PFUdS | 86.1 (5.93) | 93.1 (7.89) | 105 (10.9) | 91.0 (8.73) | 108 (6.82) |
| PFDoS | 116 (3.54) | 78.3 (8.12) | 101 (7.02) | 86.5 (9.40) | 102 (9.72) |
| PFTrDS | 95.7 (14.4) | 81.0 (2.20) | 93.3 (6.78) | 91.7 (13.2) | 106 (10.5) |
| 4:2 FTS | 103 (8.31) | 102 (12.1) | 102 (10.2) | 113 (8.30) | 116 (2.94) |
| 6:2 FTS | 108 (9.23) | 115 (6.07) | 96.4 (9.84) | 87.9 (10.2) | 109 (5.90) |
| 8:2 FTS | 92.5 (15.3) | 94.2 (12.3) | 90.5 (7.92) | 83.1 (5.62) | 95.0 (8.98) |
| FOSA | – | 103 (8.53) | 88.3 (12.9) | 96.3 (13.2) | 112 (6.15) |
| NMeFOSAA | 107 (9.71) | 86.6 (8.05) | 86.1 (8.46) | 92.4 (10.7) | 98.9 (12.9) |
| NEtFOSAA | 107 (7.37) | 94.3 (8.12) | 94.8 (10.6) | 105 (7.31) | 110 (5.73) |
| PFMPA | 86.1 (9.47) | 88.4 (8.49) | 84.0 (9.50) | 85.4 (5.55) | 90.4 (7.52) |
| PFMBA | 87.4 (10.3) | 98.5 (11.8) | 85.8 (8.29) | 98.6 (8.80) | 102 (5.72) |
| HFPO-DA | – | 98.7 (13.5) | 96.9 (9.14) | 88.2 (15.1) | 98.6 (14.0) |
| ADONA | 113 (4.56) | 87.7 (7.80) | 85.9 (7.20) | 84.5 (1.90) | 91.5 (8.03) |
| PFEESA | 94.1 (10.6) | 91.2 (12.4) | 98.4 (11.7) | 104 (8.42) | 110 (7.01) |
| 9Cl-PF3ONS | 93.2 (7.90) | 80.5 (7.82) | 83.7 (5.62) | 88.6 (5.48) | 97.2 (11.2) |
| 11Cl-PF3OUdS | 99.8 (8.00) | 82.3 (6.39) | 92.4 (9.85) | 101 (13.3) | 106 (8.15) |
| Capstone A | – | 85.9 (11.8) | 103 (9.31) | 111 (5.74) | 118 (6.80) |
| Capstone B | – | 108 (8.60) | 99.9 (15.0) | 113 (4.46) | 118 (2.83) |
Almond Milk
| Average Recovery (RSD, %), n=9 | |||||
|---|---|---|---|---|---|
| Fortified Concentration (µg/kg) | |||||
| Analytes | 0.010 | 0.025 | 0.050 | 0.10 | 0.25 |
| 13C-TFA | – | 127 (4.47) | 129 (2.78) | 125 (7.85) | 110 (8.27) |
| PFPrA | – | 120 (5.45) | 115 (6.71) | 114 (5.40) | 119 (5.62) |
| PFBA | 112 (7.95) | 106 (8.08) | 103 (8.48) | 98.0 (6.45) | 101 (14.2) |
| PFPeA | 97.1 (7.51) | 101 (10.8) | 99.5 (10.8) | 92.1 (8.40) | 95.4 (7.09) |
| PFHxA | 90.8 (12.2) | 111 (10.1) | 100 (7.90) | 94.0 (6.60) | 102 (9.41) |
| PFHpA | 116 (5.79) | 98.8 (6.45) | 88.9 (5.97) | 87.4 (6.15) | 94.1 (6.10) |
| PFOA | 109 (7.29) | 113 (6.75) | 102 (6.76) | 109 (3.37) | 114 (3.33) |
| PFNA | 88.0 (8.26) | 90.0 (7.59) | 81.6 (6.43) | 84.6 (4.29) | 89.7 (7.17) |
| PFDA | 106 (14.3) | 95.0 (7.55) | 84.7 (5.31) | 92.6 (5.61) | 94.2 (3.03) |
| PFUnA | 105 (9.34) | 93.4 (8.81) | 99.1 (11.7) | 108 (6.58) | 106 (4.40) |
| PFDoA | 115 (4.66) | 114 (3.60) | 114 (5.06) | 119 (2.60) | 119 (2.65) |
| PFTrDA | 105 (8.08) | 111 (4.47) | 116 (6.70) | 126 (3.29) | 125 (3.64) |
| PFTeDA | 106 (6.29) | 112 (3.62) | 118 (2.81) | 128 (3.46) | 128 (3.19) |
| TFMS | 100 (9.22) | 107 (7.45) | 110 (4.41) | 87.9 (5.54) | 88.9 (8.59) |
| PFEtS | 115 (6.22) | 111 (7.45) | 118 (3.40) | 112 (6.29) | 118 (3.93) |
| PFPrS | 91.9 (8.44) | 92.3 (5.94) | 100 (8.15) | 95.5 (4.92) | 98.3 (7.83) |
| PFBS | 112 (6.31) | 92.1 (11.8) | 98.9 (8.42) | 90.1 (7.68) | 101 (10.6) |
| PFPeS | 99.7 (13.6) | 111 (10.9) | 103 (7.36) | 100 (10.0) | 106 (11.5) |
| PFHxS | 100 (9.16) | 95.9 (9.88) | 101 (10.4) | 111 (6.40) | 108 (5.15) |
| PFHpS | 86.9 (6.71) | 96.0 (9.84) | 84.5 (5.45) | 92.1 (3.27) | 99.5 (5.34) |
| PFOS | 115 (4.37) | 90.3 (10.9) | 83.4 (5.20) | 83.7 (7.66) | 91.3 (8.34) |
| PFNS | 89.6 (12.1) | 92.3 (11.3) | 82.8 (5.36) | 95.0 (6.73) | 93.0 (10.6) |
| PFDS | 108 (12.8) | 101 (11.6) | 110 (5.53) | 110 (9.70) | 119 (3.14) |
| PFUdS | 93.9 (11.4) | 93.8 (9.55) | 98.4 (7.86) | 115 (1.83) | 118 (3.13) |
| PFDoS | 90.3 (7.00) | 100 (9.86) | 107 (6.95) | 118 (2.42) | 118 (2.26) |
| PFTrDS | 95.9 (10.9) | 94.0 (9.28) | 99.6 (6.13) | 114 (3.42) | 115 (3.09) |
| 4:2 FTS | 87.6 (7.37) | 108 (7.99) | 105 (8.12) | 99.7 (8.11) | 105 (8.52) |
| 6:2 FTS | 113 (6.80) | 113 (6.76) | 110 (5.34) | 105 (6.80) | 105 (5.44) |
| 8:2 FTS | 118 (3.57) | 101 (8.58) | 96.4 (7.93) | 108 (4.60) | 103 (6.98) |
| FOSA | 105 (12.5) | 103 (7.55) | 107 (6.24) | 97.7 (10.6) | 109 (5.68) |
| NMeFOSAA | 105 (5.69) | 92.2 (13.1) | 87.0 (7.11) | 91.8 (8.89) | 98.0 (8.37) |
| NEtFOSAA | 108 (7.59) | 103 (9.82) | 88.1 (9.60) | 101 (10.5) | 104 (6.61) |
| PFMPA | 88.7 (6.82) | 93.8 (9.95) | 97.8 (13.1) | 97.2 (9.27) | 109 (5.00) |
| PFMBA | 94.1 (11.1) | 90.9 (10.4) | 96.1 (9.65) | 98.9 (6.79) | 107 (6.54) |
| HFPO-DA | – | 107 (9.78) | 103 (10.2) | 105 (11.1) | 112 (5.38) |
| ADONA | 101 (13.8) | 104 (8.18) | 88.0 (7.00) | 83.8 (7.37) | 89.8 (5.29) |
| PFEESA | 103 (13.6) | 95.2 (8.88) | 97.2 (10.9) | 94.9 (7.39) | 101 (10.3) |
| 9Cl-PF3ONS | 108 (8.15) | 96.7 (3.42) | 81.7 (3.82) | 95.0 (4.48) | 94.2 (5.43) |
| 11Cl-PF3OUdS | 86.7 (8.10) | 92.9 (8.10) | 82.9 (2.81) | 85.7 (3.71) | 84.0 (9.17) |
| Capstone A | – | – | – | – | – |
| Capstone B | – | 104 (7.55) | 95.0 (14.0) | 105 (9.55) | 92.6 (11.2) |
Infant Formula
| Average Recovery (RSD, %), n=9 | |||||
|---|---|---|---|---|---|
| Fortified Concentration (µg/kg) | |||||
| Analytes | 0.010 | 0.025 | 0.050 | 0.10 | 0.25 |
| 13C-TFA | – | 95.8 (12.9) | 102 (6.25) | 110 (10.2) | 110 (9.68) |
| PFPrA | – | 96.6 (8.80) | 99.2 (7.87) | 92.0 (4.92) | 116 (4.05) |
| PFBA | 107 (7.01) | 107 (8.41) | 108 (4.84) | 110 (4.96) | 109 (4.42) |
| PFPeA | 93.1 (6.70) | 99.7 (9.51) | 99.8 (9.22) | 88.3 (7.39) | 99.3 (6.81) |
| PFHxA | 101 (8.37) | 94.5 (10.6) | 99.9 (7.70) | 98.5 (4.85) | 106 (7.37) |
| PFHpA | 94.0 (9.15) | 98.6 (8.38) | 99.6 (7.19) | 97.0 (8.02) | 104 (6.90) |
| PFOA | 97.2 (11.7) | 102 (10.9) | 103 (5.99) | 105 (6.93) | 110 (3.65) |
| PFNA | 101 (5.60) | 97.0 (5.33) | 107 (4.99) | 110 (5.07) | 116 (4.24) |
| PFDA | 91.4 (6.59) | 89.9 (6.39) | 110 (4.69) | 116 (3.48) | 113 (1.86) |
| PFUnA | 99.1 (10.8) | 86.8 (4.92) | 105 (7.70) | 116 (3.81) | 112 (7.38) |
| PFDoA | 82.7 (3.01) | 82.2 (2.66) | 96.4 (3.87) | 106 (4.87) | 106 (4.39) |
| PFTrDA | 86.2 (3.82) | 81.5 (1.67) | 96.2 (6.82) | 96.9 (4.46) | 113 (5.98) |
| PFTeDA | 81.8 (4.54) | 80.8 (6.32) | 89.8 (2.41) | 90.1 (3.28) | 106 (3.09) |
| TFMS | 110 (7.01) | 99.5 (8.05) | 99.3 (7.09) | 107 (9.75) | 106 (10.4) |
| PFEtS | 90.4 (12.1) | 94.9 (6.25) | 88.8 (5.44) | 90.0 (7.63) | 96.0 (5.53) |
| PFPrS | 99.4 (9.37) | 92.3 (8.05) | 97.1 (4.87) | 94.4 (7.57) | 101 (4.53) |
| PFBS | 101 (12.8) | 107 (8.88) | 98.2 (11.5) | 94.6 (7.03) | 109 (11.2) |
| PFPeS | 111 (7.96) | 106 (7.55) | 108 (8.38) | 106 (4.36) | 111 (6.12) |
| PFHxS | 85.5 (6.65) | 89.1 (12.3) | 96.1 (13.3) | 96.2 (9.71) | 105 (5.29) |
| PFHpS | 87.9 (9.29) | 88.0 (4.81) | 90.9 (4.84) | 89.1 (6.79) | 94.4 (3.34) |
| PFOS | 89.2 (9.65) | 89.4 (10.7) | 112 (7.14) | 106 (7.94) | 107 (6.53) |
| PFNS | 86.9 (9.81) | 82.7 (5.10) | 108 (6.65) | 107 (10.4) | 108 (5.39) |
| PFDS | 87.5 (11.9) | 88.9 (9.25) | 109 (7.57) | 106 (9.44) | 105 (8.17) |
| PFUdS | 89.7 (10.2) | 84.9 (5.13) | 105 (9.17) | 95.3 (10.4) | 97.1 (6.65) |
| PFDoS | 91.0 (9.86) | 81.7 (6.58) | 98.6 (10.6) | 84.7 (8.43) | 89.8 (9.51) |
| PFTrDS | 80.5 (6.95) | 83.6 (13.6) | 94.6 (8.68) | 84.6 (12.2) | 93.0 (13.4) |
| 4:2 FTS | 101 (10.1) | 113 (6.58) | 108 (6.31) | 112 (4.14) | 112 (6.30) |
| 6:2 FTS | 111 (7.44) | 99.8 (5.59) | 110 (9.56) | 116 (4.78) | 118 (4.57) |
| 8:2 FTS | 89.4 (8.88) | 86.2 (8.13) | 107 (6.72) | 117 (4.84) | 115 (5.38) |
| FOSA | 88.4 (8.80) | 90.0 (7.86) | 96.5 (13.6) | 99.7 (7.60) | 107 (12.2) |
| NMeFOSAA | 89.0 (10.5) | 87.9 (9.53) | 101 (9.92) | 102 (8.70) | 103 (6.85) |
| NEtFOSAA | 104 (9.71) | 91.4 (9.41) | 109 (7.39) | 115 (4.19) | 115 (4.33) |
| PFMPA | – | 82.1 (4.74) | 82.0 (3.76) | 83.1 (4.58) | 84.9 (4.63) |
| PFMBA | 88.7 (12.1) | 103 (12.5) | 96.2 (8.93) | 92.3 (9.42) | 103 (6.87) |
| HFPO-DA | – | 94.7 (13.7) | 105 (7.55) | 101 (5.29) | 104 (9.62) |
| ADONA | 117 (3.44) | 108 (8.64) | 108 (7.27) | 104 (7.23) | 108 (6.31) |
| PFEESA | 99.7 (10.9) | 110 (5.44) | 106 (6.32) | 100 (8.12) | 109 (10.2) |
| 9Cl-PF3ONS | 84.8 (5.47) | 87.8 (5.83) | 83.6 (6.65) | 84.4 (3.03) | 86.3 (4.58) |
| 11Cl-PF3OUdS | 84.2 (7.68) | 91.6 (8.41) | 106 (5.69) | 101 (7.79) | 100 (5.06) |
| Capstone A | – | 89.7 (10.4) | 81.9 (7.72) | 83.7 (6.37) | 88.4 (9.29) |
| Capstone B | – | 93.8 (11.3) | 93.6 (12.4) | 107 (11.8) | 104 (12.7) |
Real-World Sample Analysis
A total of 24 milk samples were collected from local grocery stores to assess PFAS contamination using the established workflow. Each sample was prepared in duplicate. The results revealed widespread presence of TFA, TFMS, PFBA, and PFHpA in the milk samples (Table IV). Notably, all tested soymilk samples contained high levels of PFPrA and PFBA. Long-chain compounds, including PFOA, PFOS, PFNA, PFDoA, and PFTeDA, were detected in some samples. A fluorotelomer sulfonic acid compound, 6:2 FTS, was the only PFAS identified aside from perfluoroalkyl carboxylic and sulfonic acids, and it was present in all four infant formula samples and some of the other milk samples. TFA concentrations were significantly higher and exceed the calibration range in some milk samples, necessitating sample dilution prior to extraction to obtain accurate quantification.
Table IV: Measurement of 41 Targeted PFAS in Various Milk Matrices
| Concentration (µg/kg) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ultrashort-Chain | Short-Chain | Long-Chain | Others | ||||||||
| Milk Samples | TFA | PFPrA | TFMS | PFBA | PFHpA | PFOA | PFOS | PFNA | PFDoA | PFTeDA | 6:2 FTS |
| Dairy Milk | |||||||||||
| Whole Milk #1 | 2.3 | nd | 0.0049 | nd | nd | nd | nd | nd | nd | nd | nd |
| Whole Milk #2 | 4.3 | 0.035 | 0.065 | 0.087 | 0.0093 | 0.0044 | nd | nd | nd | nd | nd |
| Whole Milk #3 | 4.1 | nd | <0.0040 | 0.038 | <0.0040 | 0.0045 | nd | nd | <0.0040 | nd | 0.011 |
| Whole Milk #4 | 3.0 | nd | 0.0052 | 0.012 | <0.0040 | nd | nd | nd | nd | nd | nd |
| 2% Reduced Fat Milk #1 | 3.5 | nd | 0.012 | 0.023 | 0.0045 | 0.0094 | 0.018 | <0.0040 | <0.0040 | <0.0040 | 0.0043 |
| 2% Reduced Fat Milk #2 | 4.0 | nd | 0.0052 | 0.015 | nd | nd | nd | nd | nd | nd | nd |
| Fat-Free Milk #1 | 3.6 | nd | 0.0055 | 0.011 | nd | nd | nd | nd | nd | nd | nd |
| Fat-Free Milk #2 | 3.4 | nd | 0.0046 | 0.010 | <0.0040 | <0.0040 | nd | nd | nd | nd | 0.0041 |
| Fat-Free Milk #3 | 2.4 | nd | 0.0076 | nd | nd | nd | nd | nd | nd | nd | nd |
| Plant-Based Milk | |||||||||||
| Almond Milk #1 | 1.8 | nd | 0.0043 | 0.017 | nd | nd | nd | nd | nd | nd | nd |
| Almond Milk #2 | 2.2 | nd | 0.0070 | 0.021 | <0.0040 | <0.0040 | nd | nd | <0.0040 | nd | <0.0040 |
| Almond Milk #3 | 5.3 | nd | 0.013 | 0.039 | <0.0040 | nd | nd | nd | nd | nd | nd |
| Oat Milk #1 | 7.2 | nd | 0.027 | 0.016 | <0.0040 | 0.0052 | 0.0086 | nd | nd | nd | 0.055 |
| Oat Milk #2 | 11 | nd | 0.021 | 0.019 | nd | nd | nd | nd | nd | nd | 0.0053 |
| Soy Milk #1 | 24 | 0.52 | 0.016 | 0.21 | <0.0040 | nd | nd | nd | nd | nd | nd |
| Soy Milk #2 | 11 | 0.26 | 0.013 | 0.10 | <0.0040 | nd | nd | nd | nd | nd | nd |
| Soy Milk #3 | 5.6 | 0.088 | 0.016 | 0.069 | nd | nd | nd | nd | nd | nd | nd |
| Coconut Milk #1 | 1.1 | nd | 0.0047 | 0.013 | <0.0040 | nd | nd | nd | nd | nd | nd |
| Coconut Milk #2 | 1.0 | nd | 0.0043 | nd | <0.0040 | 0.0053 | nd | nd | nd | nd | 0.0053 |
| Infant Formula | |||||||||||
| Formula #1 | 1.0 | nd | <0.0040 | nd | nd | nd | nd | nd | nd | nd | 0.0064 |
| Formula #2 | 0.4 | nd | <0.0040 | nd | <0.0040 | nd | nd | nd | nd | nd | 0.0074 |
| Formula #3 | 0.8 | nd | <0.0040 | 0.012 | <0.0040 | <0.0040 | nd | nd | nd | nd | 0.0050 |
| Formula #4 | 1.2 | nd | 0.0069 | 0.011 | <0.0040 | 0.0050 | nd | nd | nd | nd | 0.012 |
| nd = not detected | |||||||||||
Conclusion
This study developed a simple, robust workflow for comprehensive PFAS analysis in various liquid milk matrices, successfully incorporating ultrashort-chain PFAS along with more commonly analyzed compounds. The optimized sample preparation protocol, combined with a sensitive LC method, enabled effective extraction and quantification of 41 PFAS with high accuracy and precision. The LC method leveraged a distinctive Ultra Inert IBD column, which features a polar-embedded alkyl stationary phase and an inert surface coating, providing enhanced retention for ultrashort-chain compounds and minimized analyte interaction to achieve increased sensitivity. Application of this workflow to commercial milk samples provided valuable insights into PFAS contamination across different milk sources. These findings highlight the importance of integrating ultrashort-chain PFAS into routine monitoring of PFAS in food products and offer a practical approach to support future food safety research and regulatory initiatives.
References
- J. York, Analysis of PFAS in milk by LC-MS/MS, Application note, FSAN4338-UNV, Restek Corporation, 2024. https://www.restek.com/articles/analysis-of-pfas-in-milk-by-lc-ms-ms
- S.H. Liang, Incorporating ultrashort-chain compounds into comprehensive PFAS analysis in waters, Application note, EVAN4402-UNV, Restek Corporation, 2025. https://www.restek.com/articles/incorporating-ultrashort-chain-compounds-into-comprehensive-pfas-analysis-in-waters
- S.H. Liang, J. Steimling, C1-C10 PFAS analysis in human plasma and serum, Application note, CFAN4273A-UNV, Restek Corporation, 2025. https://www.restek.com/articles/c1-c10-pfas-analysis-in-human-plasma-and-serum
- European Union Reference Laboratory for halogenated POPs in Feed and Food. Guidance Document on Analytical Parameters for the Determination of Per- and Polyfluoroalkyl Substances (PFAS) in Food and Feed. Version 1.2. May 2022. https://eurl-pops.eu/news/guidance-document-pfas/guidance-document-pfas