In this blog we’ll continue looking into individual columns and specific separations. We’ll focus on Rt-βDEXsa, Rt-βDEXcst and Rt-γDEXsa (Table 1), which all have somewhat different selectivity from the columns discussed in the last blog (Rt-βDEXm, Rt-βDEXsm and Rt-βDEXse).
Table 1: Resolution of selected chiral compounds by Rt-βDEXsa, Rt-βDEXcst and Rt-γDEXsa
| Rt-ßDEXsa | Rt-ßDEXcst | Rt-γDEXsa | |
| 1-octen-3-ol | 2.00 | ns | 1.35 |
| β-citronellol | 0.98 | ns | ns |
| menthol | 0.93 | 0.89 | 2.89 |
| camphor | 4.20 | 2.22t | 3.64 |
| α-ionone | 4.69 | 1.37 | 8.43 |
| γ-nonalactone | 4.00 | 3.82 | 0.99 |
| γ-undecalactone | 3.65 | 3.18 | 0.84 |
| δ-dodecalactone | 1.91 | 1.69 | 0.75 |
Chiral Separations with Rt-βDEXsa
The Rt-bDEXsa has a significantly different selectivity than the other chiral columns. It provides the best separation of 1-octen-3-ol, carvone, camphor, b-citronellol (Figure 1A-D, respectively), and rose oxides (Figure 2).
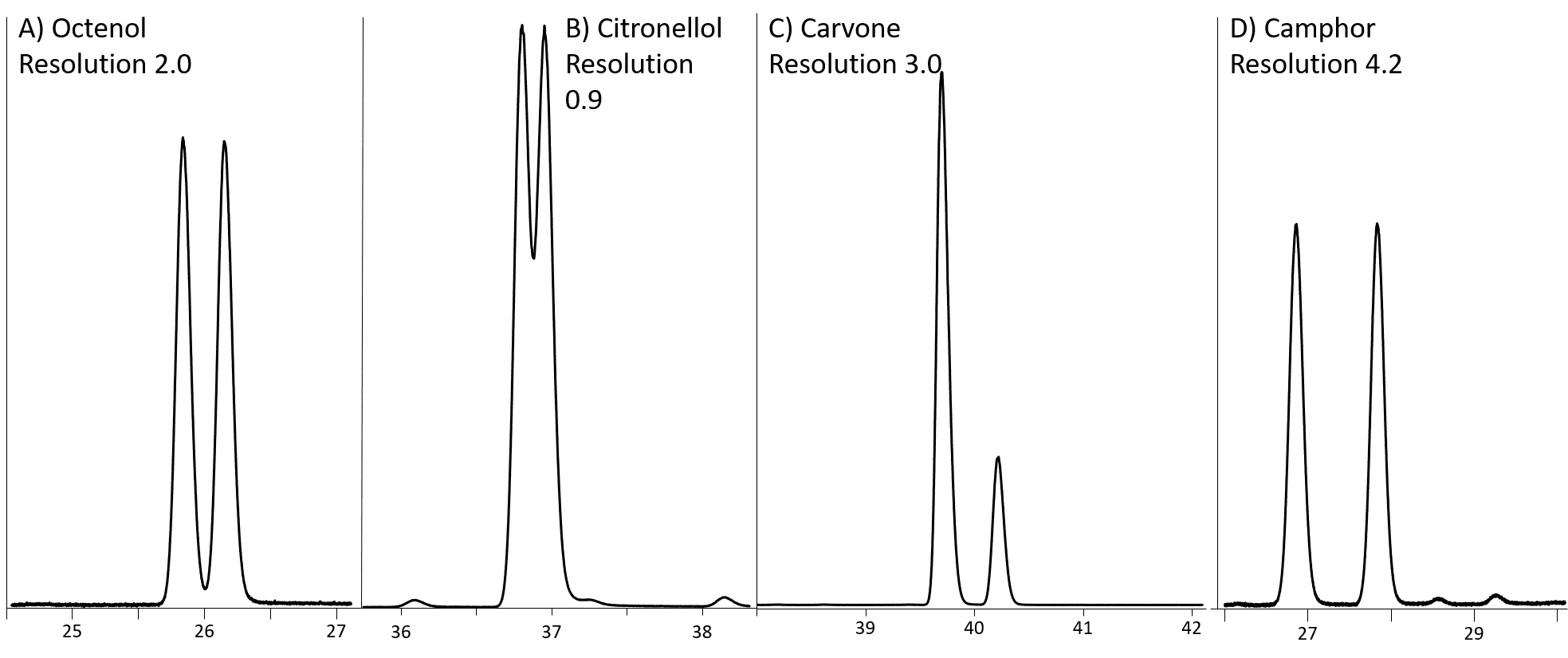
Figure 1: Separation of A) 1-octen-3-ol, B) β-citronellol, C) carvone and D) Camphor on Rt-bDEXsa
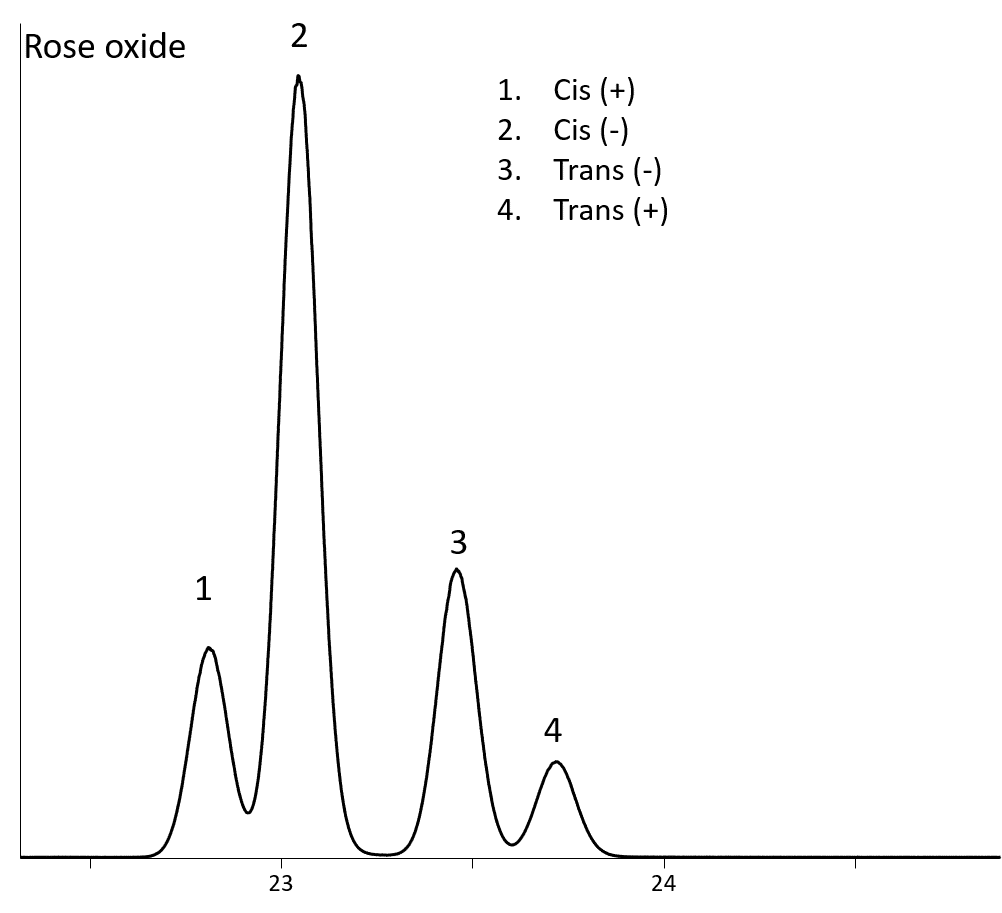
Figure 2: Separation of Rose oxides on Rt-bDEXsa
Chiral Separation with Rt-ßDEXcst
This column is optimum for semivolatile chiral compounds. It provides good resolution of the irone isomers (found in iris flowers; Figure 3). This is also suitable column for resolution of the γ- and δ-lactones (Figure 4).
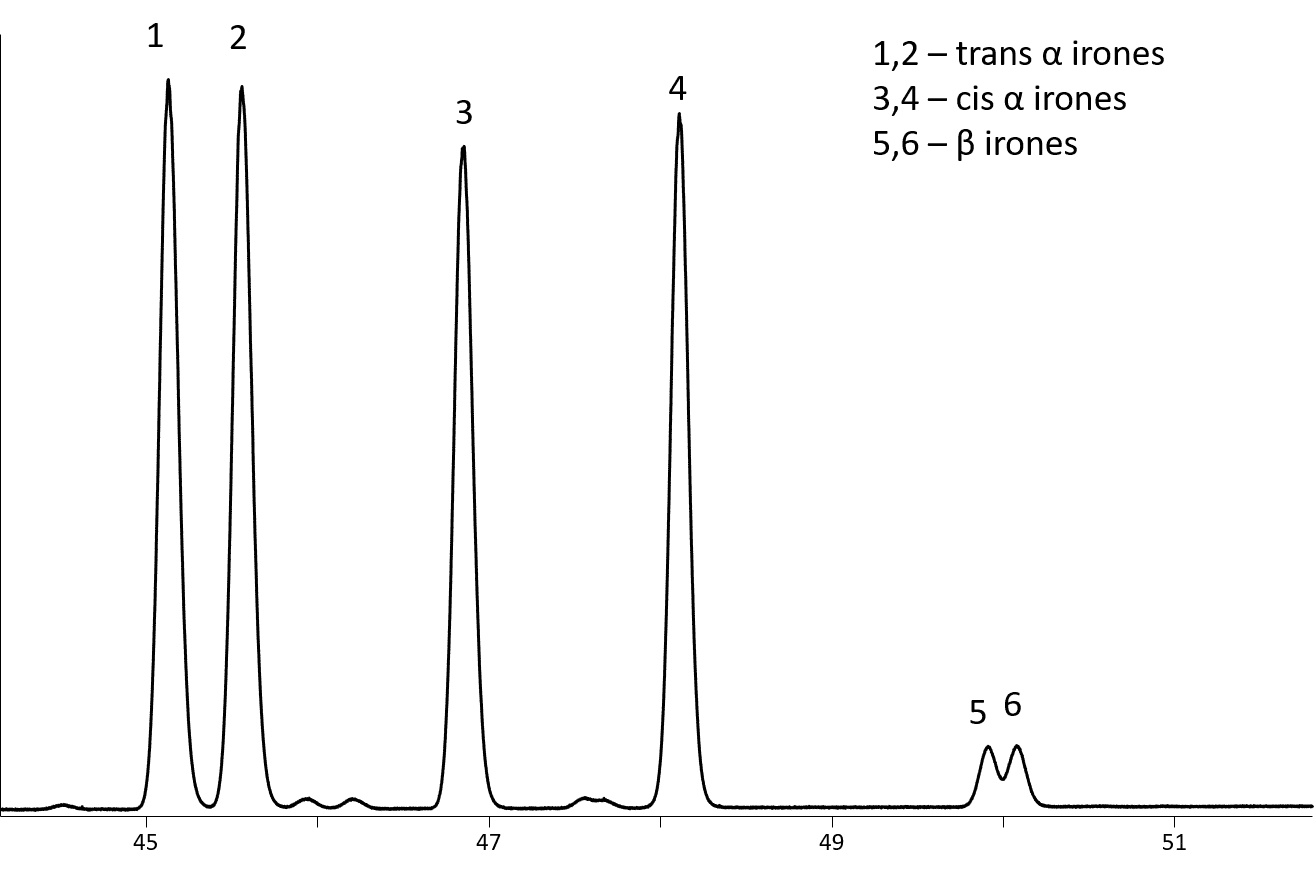
Figure 3: Separation of irone isomers on Rt-ßDEXcst
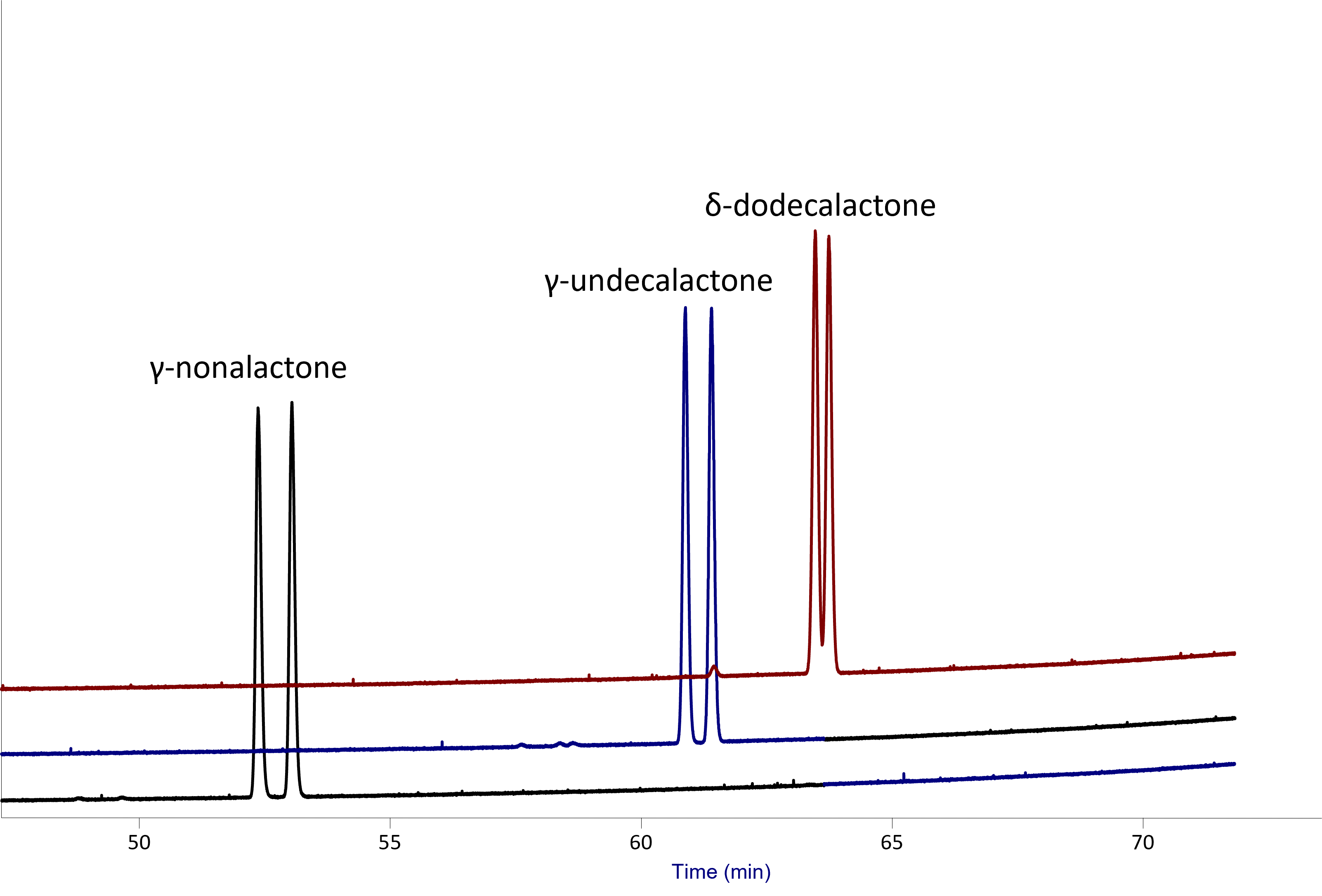
Figure 4: Separation of lactones on Rt-ßDEXcst
Chiral Separations with Rt-γDEXsa
The Rt-γDEXsa is unique in the Restek chiral column selection due to doping of γ-cyclodextrins into the stationary phase polymer. The γ-cyclodextrins have a larger cavity; therefore, this column is useful for larger organic molecules. While our study was focused more on small molecules, the Rt-γDEXsa had very good separation for menthol and α-ionone (Figure 5).
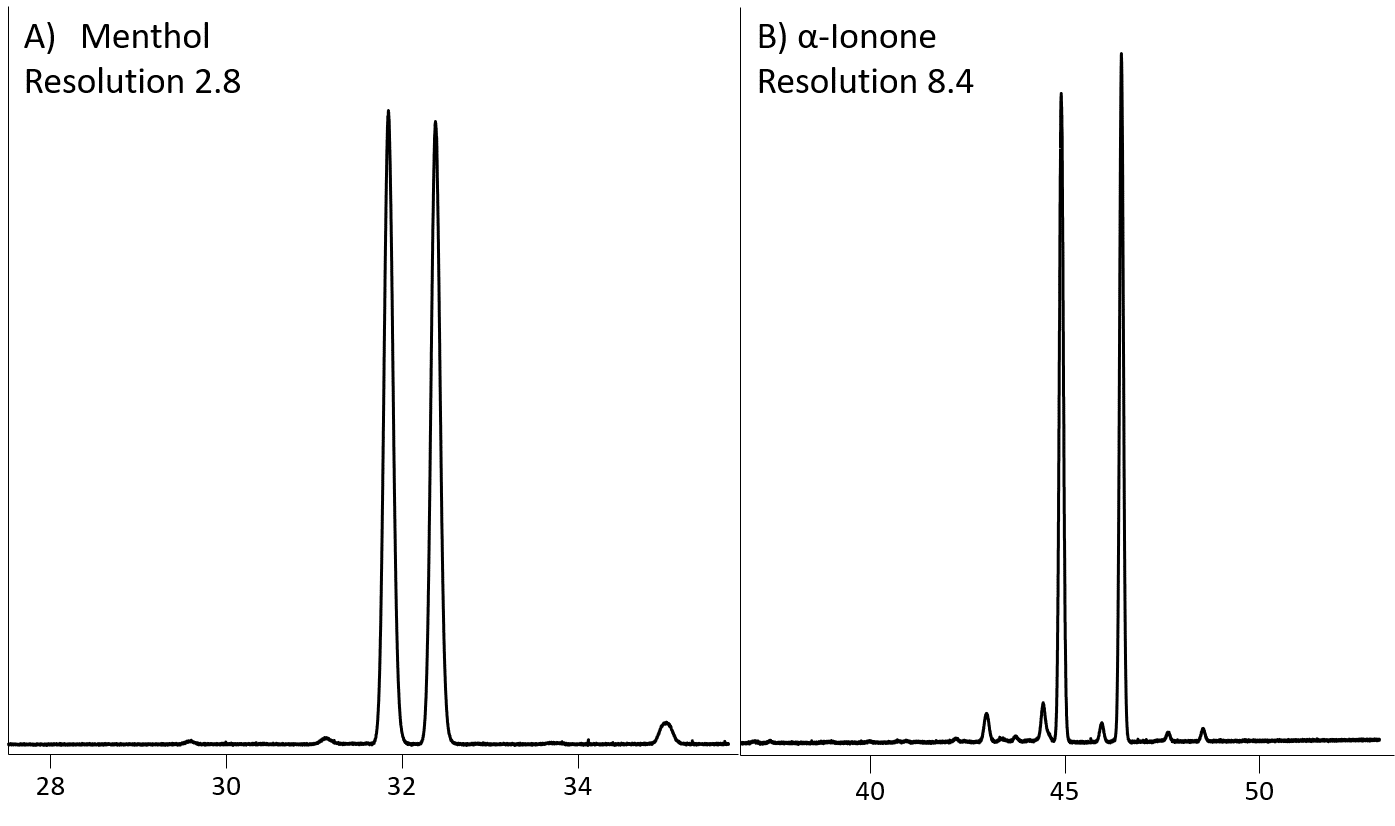
Figure 5: Separation of A) menthol and B) α-Ionone on Rt- γDEXsa



