Supported liquid extraction (SLE) is an easy-to-use and beneficial sample clean up technique. This preparation is a scaled down version of liquid-liquid extraction and can be used to remove unwanted materials such as salts, proteins, and phospholipids from samples. SLE works by loading an aqueous sample onto the cartridge or plate and waiting for 5 minutes to allow the sample to interact with the material so that small water droplets can be dispersed across the extraction bed. After this occurs, analytes of interest can be extracted using a water immiscible organic solvent. The salts, proteins, and phospholipids remain in the water droplets on the material while the analytes of interest partition into the nonpolar extraction solvent, yielding a cleaner sample. Supported liquid extraction is highlighted in an application note comparing different sample preparation techniques for drugs of abuse in oral fluids. The full application note can be found here.
One of the most difficult aspects of SLE, and the focus of this blog, is finding an elution solvent that is polar enough to elute the analytes of interest, while still retaining matrix components. Using highly polar solvents for elution will improve the recovery of more polar analytes from the SLE bed, however it may leave you with dirtier samples. An additional element to consider with SLE is solvent miscibility. Ideally, the solvents used in SLE should be immiscible. This allows for the partitioning of analytes based on their solubility in each phase. In Table I below, different elution solvents were tested on analytes from a range of drug classes and properties.
Table I: Comparison of Elution Solvents on Analyte Peak Area
| Analyte | Hydromorphone (RT = 1.4 min) |
Levamisole (RT = 3.0 min) |
Benzoylecgonine (RT = 4.2 min) |
Fentanyl (RT = 5.3 min) |
Oxazepam (RT = 6.7 min) |
| Elution Solvent | Peak Area | ||||
| DCM | 20400 | 70500 | 112000 | 117000 | 123000 |
| 50:50 DCM:EtOAc | 7820 | 50600 | 11300 | 84700 | 84000 |
| 95:5 DCM:IPA | 18000 | 78900 | 137000 | 137000 | 112000 |
| 95:5 DCM:MeOH | 15100 | 50000 | 71900 | 71300 | 66000 |
| Hexane | 147 | 43500 | 2350 | 173000 | 1020 |
| ACN | 4710 | 9120 | 28400 | 15900 | 13700 |
| MeOH | 40300 | 56000 | 73700 | 39200 | 33800 |
These elution solvents had a range of polarities and miscibility with water. For example, methanol and acetonitrile are both solvents that are miscible with water and have a high polarity. These solvents worked well for more polar analytes such as hydromorphone, but they also resulted in dirtier samples. Hexane is much more non-polar and is water immiscible. However, hexane returns much lower recovery for most analytes aside from levamisole and fentanyl. A mixture of 95:5 DCM: IPA, which is highlighted in the table above, averaged the best results for this list of analytes.
To demonstrate the effects on an extremely polar analytes, hydromorphone was eluted using three different solvents. In the examples below, Figure 1 shows an elution with a more polar solvent, methanol, whereas Figure 3 shows a less polar solvent, a mixture of 95:5 Dichloromethane (DCM): Isopropyl alcohol (IPA) (v/v), yielding a cleaner sample. Figure 2 used a double elution step where 95:5 DCM: IPA was used as the first elution solvent, then the samples were eluted a second time using methanol.
Figure 1: Sample Eluted with Methanol
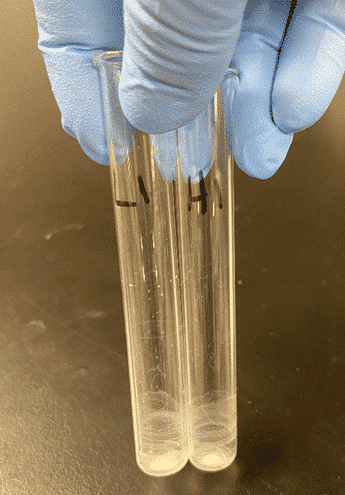
Figure 2: Sample Eluted with 1st Eluent 95:5 DCM: IPA and 2nd Eluent Methanol
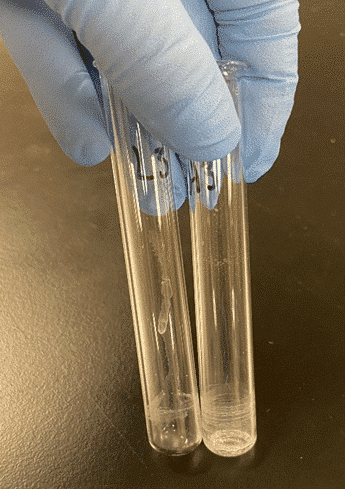
Figure 3: Sample Eluted with 95:5 DCM: IPA

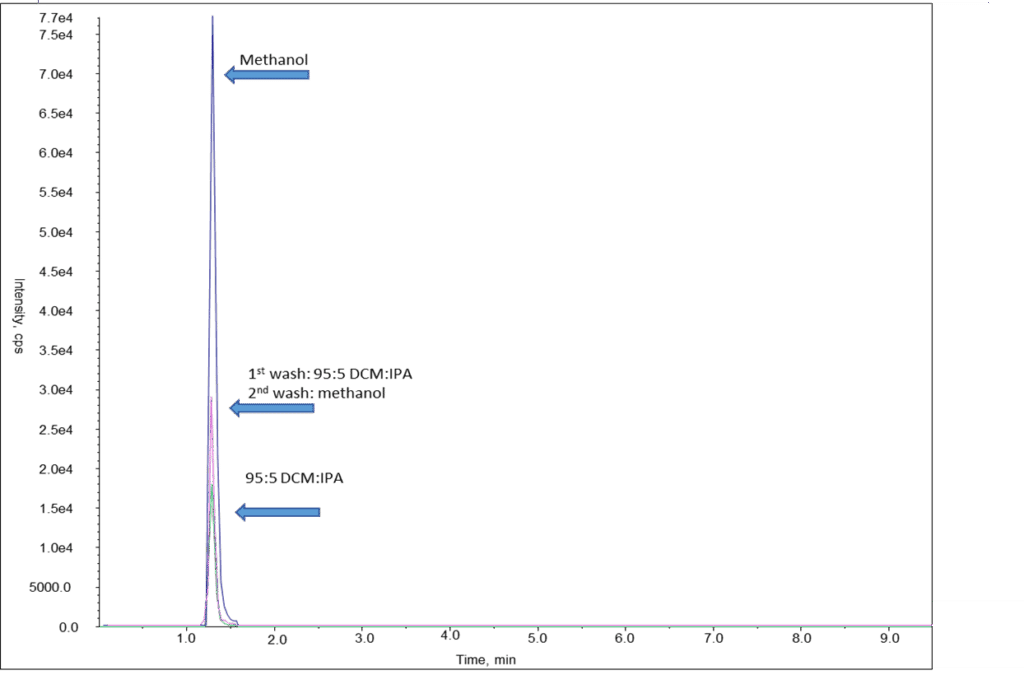
Based on the photos above, 95:5 DCM: IPA clearly yielded the cleanest results. While cleanliness of the sample is important, it is also important to get the best recovery possible for your analytes. In the examples below, hydromorphone was eluted using 95:5 DCM: IPA, methanol, and a double elution step using 95:5 DCM: IPA as the first eluent and methanol as the second. The analyte recovery can be seen in Figure 4 below. When compared, the higher polar solvent (methanol) resulted in the best sensitivity for hydromorphone.
Figure 4: Comparison of Different Polarity Elution Buffers on Hydromorphone, Overlaid
Overall, SLE can be a great solution if you are looking for more sample clean up but still want something simple and efficient. To get the best performance out of this sample preparation, it is important to find the right balance of polarity and miscibility for the elution solvent. Making small changes to the elution solvent’s polarity can yield large differences in cleanliness of the sample and sensitivity on the instrument. For more information on SLE, please see the following PDF.




