Our new inert LC column technology helps labs improve their analysis of metal-sensitive compounds. A premium inert coating applied to the stainless-steel surface of our LC columns reduces nonspecific binding of chelating analytes, enabling sensitive analysis and smooth integration of peaks. Combined with Restek’s selective stationary phases, these new inert LC columns are ideal for the analysis of metal-sensitive compounds, such as organophosphorus pesticides and mycotoxins.
Restek’s inert LC columns provide four key benefits:
- Improved peak shape without passivation or mobile phase additives.
- Increased response and analyte recovery, allowing lower detection limits.
- High accuracy and throughput with less variability.
- Less time-consuming conditioning and complicated passivation required.
Exceptional Inertness Brings Exceptional Performance
Analyzing compounds that have nonspecific adsorption (NSA) or nonspecific binding (NSB) to metal surfaces in LC columns has historically been a challenge. Poor peak shape and sensitivity are key indicators that polar, usually acidic, compounds are interacting with the metal surfaces in the column, causing poor data quality. Our premium inert column technology is designed to eliminate NSA and NSB of active analytes to the column hardware, giving analysts greater confidence in their data when working with metal-sensitive analytes.
Pair Your Inert LC Column with an Inert Guard Column for Even Greater Benefits
Available in a variety of stationary phases, our inert guard columns provide optimal protection to your LC column and system while also reducing nonspecific binding of chelating analytes. Our standalone guard columns can be used in conjunction with any LC column and are specifically designed to complement and enhance the performance of our inert columns. Go to page 11 to see the improved performance when using an inert LC column and guard compared to a conventional stainless-steel column and guard.
An Extensive Product Line to Meet Your Unique Needs
Restek’s inert LC column technology is available in various column types to accommodate a wide range of applications and testing methodologies. Our inert columns are now available for the following phase types: Raptor Biphenyl, Raptor C18, Raptor ARC-18, Force Biphenyl, and Force C18. The following columns are recommended for these specific applications:
| Inert LC Column | Application |
| Raptor Inert ARC-18 | Pesticides |
| Raptor Inert Biphenyl | Mycotoxins |
| Force Inert C18 | Methylmalonic acid |
| Raptor Inert C18 | Veterinary Drugs |
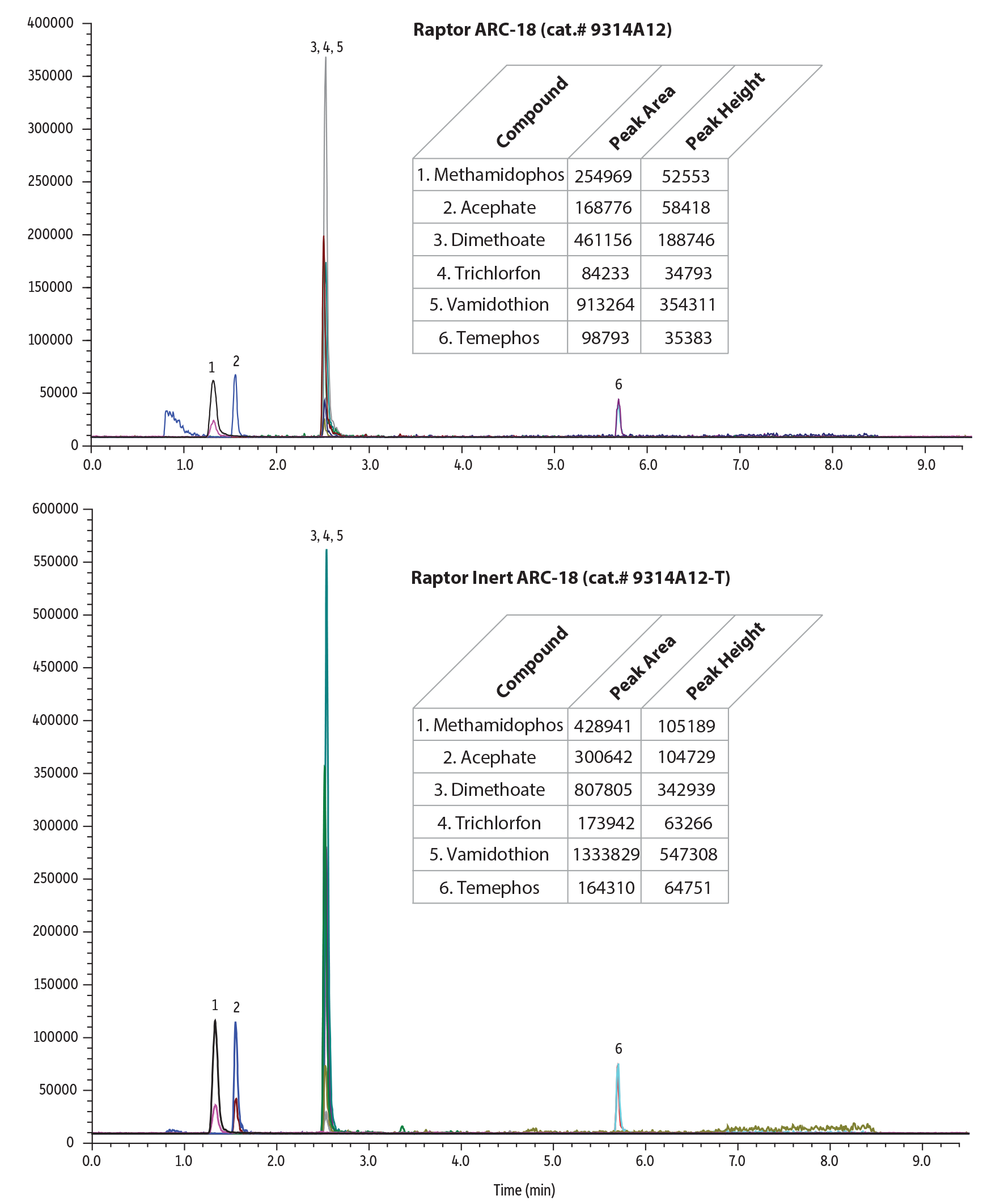
Pesticides
Pesticide panels benefit from the use of inert LC columns as they contain a wide variety of compounds. Phosphorylated, acidic, polar compounds, and/or metal chelating species, such as organophosphate pesticides, are reactive to the metal surfaces inside of the analytical column. Our new inert LC columns solve that problem easily to improve the overall performance of your pesticide panel.
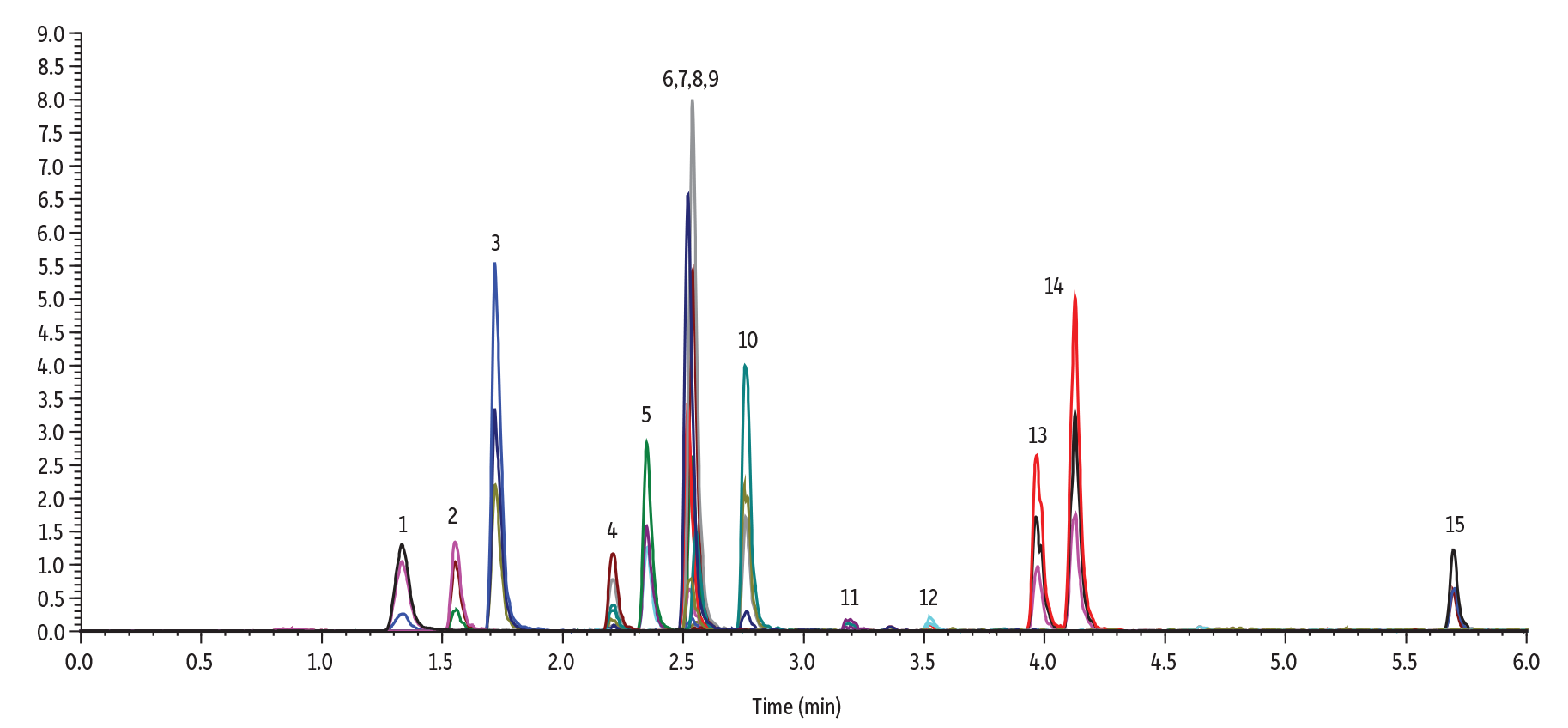
LC_EV0596
Peaks
| Peaks | tR (min) | Precursor Ion | Product Ion 1 | Product Ion 2 | Peak Area | Peak Height | |
|---|---|---|---|---|---|---|---|
| 1. | Methamidophos | 1.33 | 142.0 | 94.0 | 125.1 | 428941 | 105189 |
| 2. | Acephate | 1.55 | 184.0 | 143.0 | 48.9 | 300642 | 104729 |
| 3. | Omethoate | 1.72 | 214.0 | 125.0 | 182.9 | 892008 | 337690 |
| 4. | Monocrotophos | 2.21 | 224.1 | 127.0 | 193.1 | 215810 | 78425 |
| 5. | Dicrotophos | 2.35 | 238.1 | 112.1 | 72.0 | 404916 | 159292 |
| 6. | Dimethoate | 2.52 | 230.0 | 125.0 | 199.0 | 807805 | 342939 |
| 7. | Trichlorfon | 2.53 | 257.0 | 108.9 | 220.8 | 173942 | 63266 |
| 8. | Vamidothion | 2.54 | 288.0 | 146.0 | 118.0 | 1333829 | 547308 |
| 9. | Mevinphos isomer 1 | 2.55 | 241.9 | 126.9 | 192.9 | 311274 | 129961 |
| 10. | Mevinphos isomer 2 | 2.76 | 241.9 | 126.9 | 192.9 | 74030 | 29802 |
| 11. | Carbaryl | 3.18 | 202.1 | 145.0 | 127.0 | 39671 | 11924 |
| 12. | Isocarbophos | 3.52 | 291.1 | 231.1 | 121.1 | 33294 | 11941 |
| 13. | Dimethomorph isomer 1 | 3.96 | 388.2 | 300.9 | 165.1 | 511766 | 172977 |
| 14. | Dimethomorph isomer 2 | 4.13 | 388.2 | 300.9 | 165.1 | 877031 | 328826 |
| 15. | Temephos | 5.70 | 467.1 | 124.9 | 418.9 | 164310 | 64751 |
Conditions
| Column | Raptor Inert ARC-18 (cat.# 9314A12-T) | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||||||||||
| Temp.: | 50 °C | ||||||||||||||||||||||||||||||||
| Standard/Sample | LC multiresidue pesticide standard #1 (cat.# 31972) | ||||||||||||||||||||||||||||||||
| Diluent: | Water, 0.1% formic acid | ||||||||||||||||||||||||||||||||
| Conc.: | 1 ng/mL | ||||||||||||||||||||||||||||||||
| Inj. Vol.: | 5 µL | ||||||||||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||||||||||
| A: | Water, 2 mM ammonium formate, 0.1% formic acid | ||||||||||||||||||||||||||||||||
| B: | Methanol, 2 mM ammonium formate, 0.1% formic acid | ||||||||||||||||||||||||||||||||
|
| Detector | Shimadzu LCMS-8060 |
|---|---|
| Ion Mode: | ESI+ |
| Mode: | MRM |
| Instrument | Shimadzu Nexera X2 |
LC_EV0591
Peaks
| Peaks | Precursor Ion | Product Ion 1 | Product Ion 2 | |
|---|---|---|---|---|
| 1. | Methamidophos | 142.0 | 94.0 | 125.1 |
| 2. | Acephate | 184.0 | 143.0 | 48.9 |
| 3. | Dimethoate | 230.0 | 125.0 | 199.0 |
| 4. | Trichlorfon | 257.0 | 108.9 | 220.8 |
| 5. | Vamidothion | 288.0 | 146.0 | 118.0 |
| 6. | Temephos | 467.1 | 124.9 | 418.9 |
Conditions
| Column | See notes. | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||||||||||
| Temp.: | 50 °C | ||||||||||||||||||||||||||||||||
| Standard/Sample | LC multiresidue pesticide standard #1 (cat.# 31972) | ||||||||||||||||||||||||||||||||
| Diluent: | Water, 0.1% formic acid | ||||||||||||||||||||||||||||||||
| Conc.: | 1 ng/mL | ||||||||||||||||||||||||||||||||
| Inj. Vol.: | 5 µL | ||||||||||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||||||||||
| A: | Water, 2 mM ammonium formate, 0.1% formic acid | ||||||||||||||||||||||||||||||||
| B: | Methanol, 2 mM ammonium formate, 0.1% formic acid | ||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
| Max Pressure: | 258 bar |
| Detector | Shimadzu 8060 |
|---|---|
| Ion Mode: | ESI+ |
| Mode: | MRM |
| Instrument | Shimadzu Nexera X2 |
| Notes | Columns are: • Raptor Inert ARC-18 (cat.# 9314A12-T) • Raptor ARC-18 (cat.# 9314A12) |
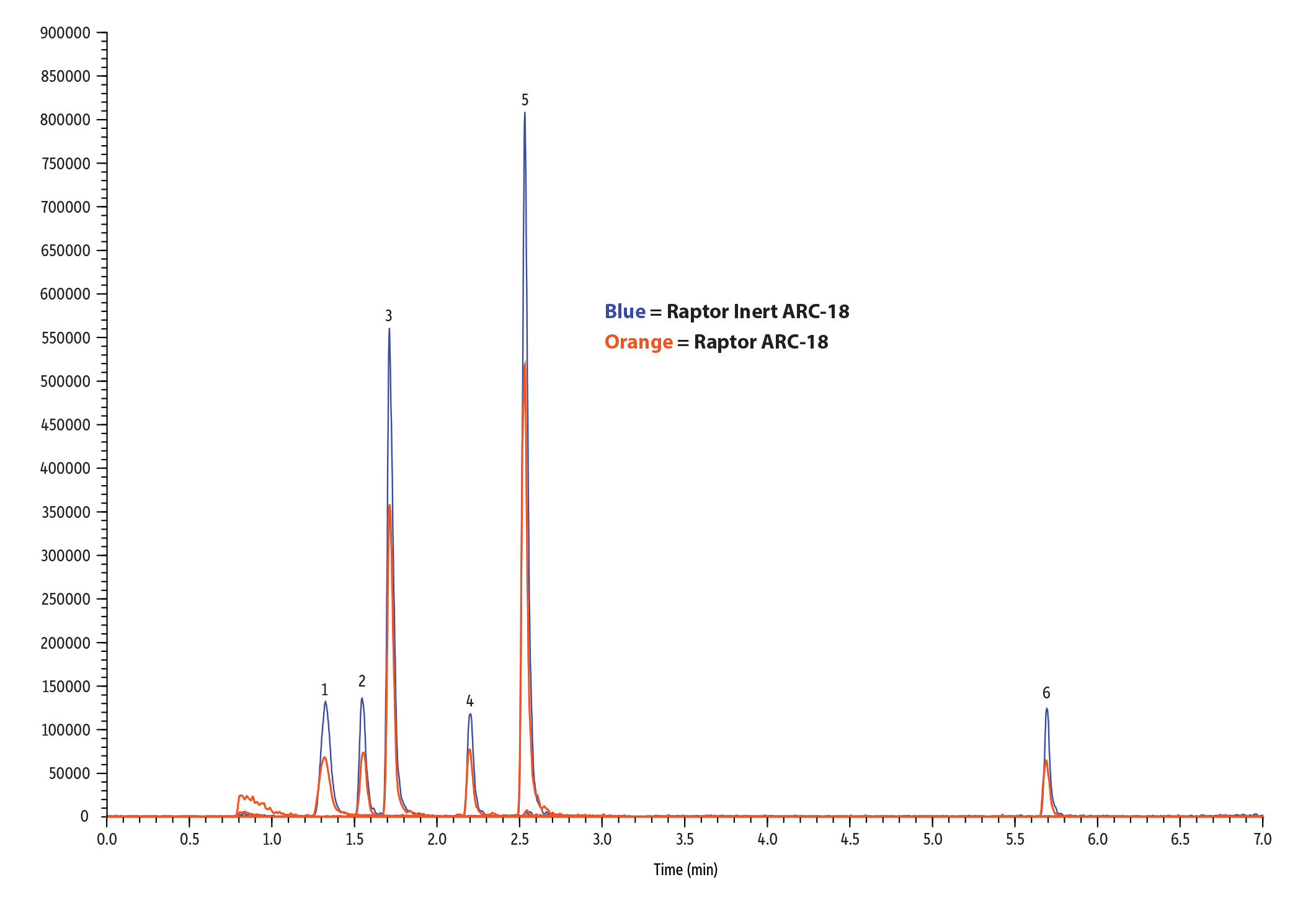
LC_EV0593
Peaks
| Peaks | Precursor Ion | Product Ion 1 | Product Ion 2 | |
|---|---|---|---|---|
| 1. | Methamidophos | 142.0 | 94.0 | 125.1 |
| 2. | Acephate | 184.0 | 143.0 | 48.9 |
| 3. | Omethoate | 214.0 | 125.0 | 182.9 |
| 4. | Monocrotophos | 224.1 | 127.0 | 193.1 |
| 5. | Vamidothion | 288.0 | 146.0 | 118.0 |
| 6. | Temephos | 467.1 | 124.9 | 418.9 |
Conditions
| Column | See notes. | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||||||||||
| Standard/Sample | LC multiresidue pesticide standard #1 (cat.# 31972) | ||||||||||||||||||||||||||||||||
| Conc.: | 1 ng/mL | ||||||||||||||||||||||||||||||||
| Inj. Vol.: | 5 µL | ||||||||||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||||||||||
| A: | Water, 2 mM ammonium formate, 0.1% formic acid | ||||||||||||||||||||||||||||||||
| B: | Methanol, 2 mM ammonium formate, 0.1% formic acid | ||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
| Max Pressure: | 258 bar |
| Detector | Shimadzu 8060 |
|---|---|
| Ion Mode: | ESI+ |
| Mode: | MRM |
| Instrument | Shimadzu Nexera X2 |
| Notes | Columns are: • Raptor Inert ARC-18 (cat.# 9314A12-T) • Raptor ARC-18 (cat.# 9314A12) |
Table I: Restek’s new inert columns showed up to 2x improvement in peak area and peak height over stainless-steel columns in this analysis of pesticides.
| Compound | Peak Area | Peak Height | ||||
| Stainless Steel | Inert | Areas Ratio (Inert/Stainless Steel) | Stainless Steel | Inert | Height Ratio (Inert/Stainless Steel) | |
| Methamidophos | 254969 | 428941 | 1.68 | 52553 | 105189 | 2.00 |
| Acephate | 168776 | 300642 | 1.78 | 58418 | 104729 | 1.79 |
| Omethoate | 579502 | 892008 | 1.54 | 216157 | 337690 | 1.56 |
| Monocrotophos | 140095 | 215810 | 1.54 | 51402 | 78425 | 1.53 |
| Dicrotophos | 340978 | 404916 | 1.19 | 135380 | 159292 | 1.18 |
| Dimethoate | 461156 | 807805 | 1.75 | 188746 | 342939 | 1.82 |
| Trichlorfon | 84233 | 173942 | 2.07 | 34793 | 63266 | 1.82 |
| Vamidothion | 913264 | 1333829 | 1.46 | 354311 | 547308 | 1.54 |
| Mevinphos isomer 1 | 213632 | 311274 | 1.46 | 82105 | 129961 | 1.58 |
| Mevinphos isomer 2 | 56093 | 74030 | 1.32 | 29070 | 29802 | 1.03 |
| Carbaryl | 43590 | 39671 | 0.91 | 14563 | 11924 | 0.82 |
| Isocarbophos | 21587 | 33294 | 1.54 | 9062 | 11941 | 1.32 |
| Dimethomorph isomer 1 | 462425 | 511766 | 1.11 | 166990 | 172977 | 1.04 |
| Dimethomorph isomer 2 | 896109 | 877031 | 0.98 | 311657 | 328826 | 1.06 |
| Temephos | 98793 | 164310 | 1.66 | 35383 | 64751 | 1.83 |
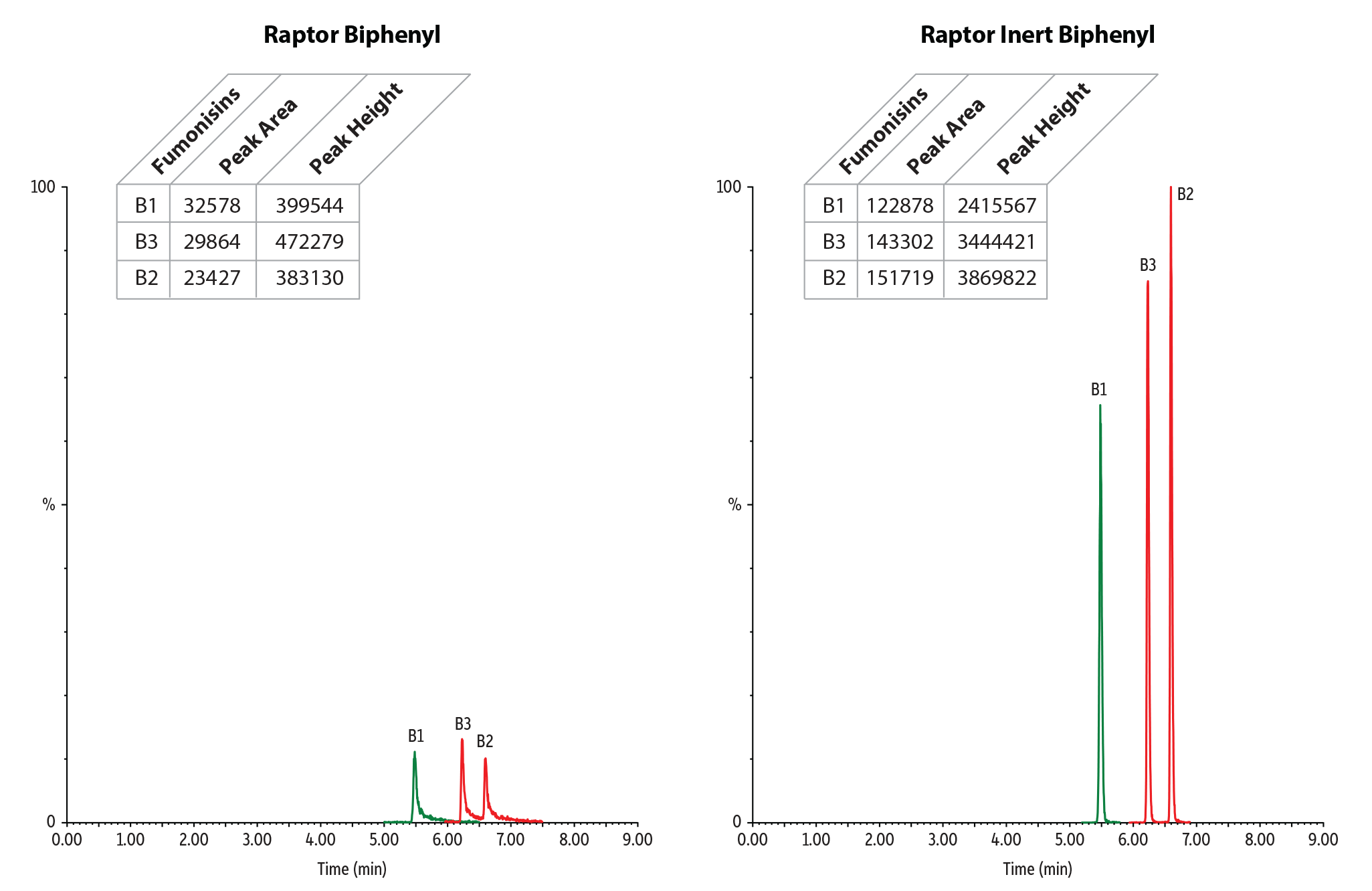
Mycotoxins
Mycotoxins analysis can be challenging and often requires a great deal of column conditioning and equilibration to achieve acceptable peaks. This is due to the reactive nature of the compounds, which contain acidic, polar, or otherwise metal chelating groups. Our new inert column hardware, combined with our stationary phases, helps simplify methods and improve the response and peak shape of these compounds.
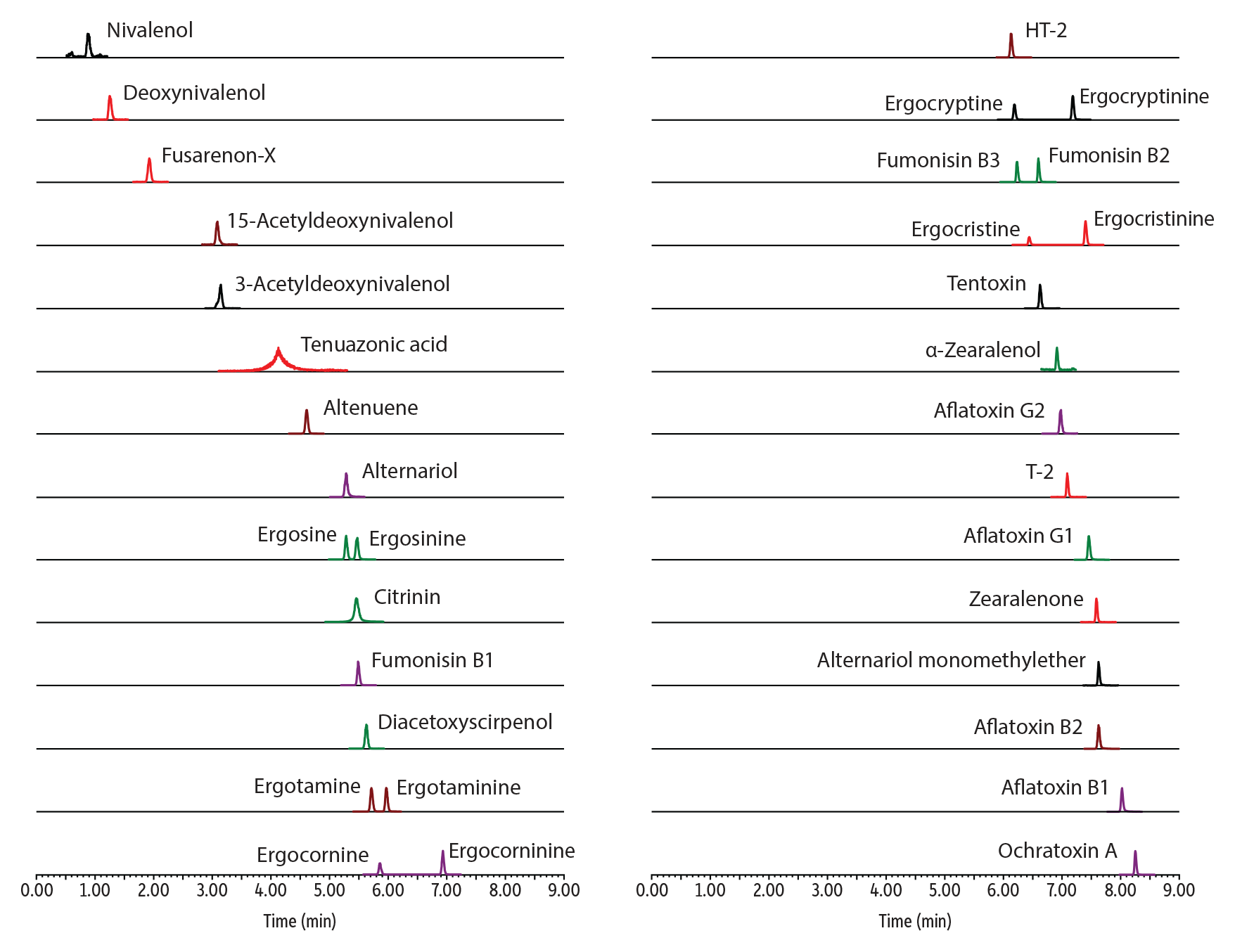
LC_FS0552
Peaks
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Peak Area | Peak Height | |
|---|---|---|---|---|---|---|---|
| 1. | Nivalenol | 0.88 | 10 | 295.1 | 137.1 | 4182 | 64495 |
| 2. | Deoxynivalenol | 1.25 | 10 | 297.2 | 231.0 | 17346 | 281906 |
| 3. | Fusarenon-X | 1.92 | 10 | 355.1 | 137.1 | 7668 | 121790 |
| 4. | 15-Acetyldeoxynivalenol | 3.08 | 10 | 339.2 | 137.1 | 31369 | 517570 |
| 5. | 3-Acetyldeoxynivalenol | 3.14 | 10 | 339.2 | 213.1 | 22613 | 296396 |
| 6. | Tenuazonic acid | 4.11 | 10 | 198.1 | 125.0 | 47828 | 197658 |
| 7. | Altenuene | 4.60 | 10 | 293.2 | 257.1 | 113850 | 2059699 |
| 8. | Alternariol | 5.27 | 10 | 259.0 | 185.1 | 73272 | 1302192 |
| 9. | Ergosine | 5.28 | 10 | 548.4 | 208.1 | 486620 | 9366601 |
| 10. | Citrinin | 5.46 | 10 | 251.2 | 233.1 | 1007880 | 9828889 |
| 11. | Ergosinine | 5.46 | 10 | 548.4 | 208.1 | 496734 | 8740527 |
| 12. | Fumonisin B1 | 5.48 | 10 | 722.5 | 352.3 | 122878 | 2415567 |
| 13. | Diacetoxyscirpenol | 5.62 | 10 | 384.2 | 247.1 | 68139 | 1208825 |
| 14. | Ergotamine | 5.71 | 10 | 582.4 | 223.2 | 493003 | 9274155 |
| 15. | Ergocornine | 5.85 | 10 | 562.4 | 268.2 | 387025 | 7732744 |
| 16. | Ergotaminine | 5.96 | 10 | 582.4 | 223.2 | 462119 | 9237991 |
| 17. | HT-2 | 6.13 | 10 | 447.2 | 345.1 | 15221 | 323765 |
| 18. | Ergocryptine | 6.19 | 10 | 576.4 | 268.2 | 522204 | 11360838 |
| 19. | Fumonisin B3 | 6.23 | 10 | 706.4 | 336.2 | 143302 | 3444421 |
| 20. | Ergocristine | 6.44 | 10 | 610.4 | 223.2 | 195562 | 4450058 |
| 21. | Fumonisin B2 | 6.59 | 10 | 706.4 | 336.2 | 151719 | 3869822 |
| 22. | Tentoxin | 6.62 | 10 | 415.2 | 312.2 | 95175 | 2131906 |
| 23. | α-Zearalenol | 6.91 | 10 | 303.1 | 285.1 | 30224 | 702420 |
| 24. | Ergocorninine | 6.93 | 10 | 562.4 | 268.2 | 704029 | 14389283 |
| 25. | Aflatoxin G2 | 6.97 | 10 | 331.2 | 189.0 | 262824 | 5274353 |
| 26. | T-2 | 7.09 | 10 | 489.2 | 387.1 | 56535 | 1394735 |
| 27. | Ergocryptinine | 7.18 | 10 | 576.4 | 268.2 | 778972 | 16765348 |
| 28. | Ergocristinine | 7.40 | 10 | 610.4 | 223.2 | 1583053 | 32975663 |
| 29. | Aflatoxin G1 | 7.45 | 10 | 329.1 | 199.7 | 304389 | 6102959 |
| 30. | Zearalenone | 7.59 | 10 | 319.2 | 283.1 | 37162 | 927455 |
| 31. | Alternariol monomethylether | 7.62 | 10 | 273.0 | 199.1 | 31024 | 640689 |
| 32. | Aflatoxin B2 | 7.63 | 10 | 315.1 | 287.0 | 295648 | 5724754 |
| 33. | Aflatoxin B1 | 8.02 | 10 | 313.2 | 241.1 | 223520 | 4425821 |
| 34. | Ochratoxin A | 8.25 | 10 | 404.1 | 239.0 | 190060 | 4411953 |
Conditions
| Column | Raptor Inert Biphenyl (cat.# 9309A12-T) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||
| Temp.: | 60 °C | ||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||
| Aflatoxins standard (cat.# 34121) | |||||||||||||||||||||||||
| Ochratoxin A standard (cat.# 34122) | |||||||||||||||||||||||||
| Diluent: | 50:50 Water:methanol | ||||||||||||||||||||||||
| Conc.: | 10 ng/mL | ||||||||||||||||||||||||
| Inj. Vol.: | 5 µL | ||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||
| A: | Water, 0.05% formic acid | ||||||||||||||||||||||||
| B: | Methanol, 0.05% formic acid | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Max Pressure: | 440 bar |
| Detector | Waters Xevo TQ-S |
|---|---|
| Ion Mode: | ESI+ |
| Mode: | MRM |
| Instrument | Waters ACQUITY UPLC I-Class |
| Notes |
Table II: In this analysis of mycotoxins, our new inert columns can provide an up to 10x increase in peak height over conventional columns.
| Compound | Peak Area | Peak Height | ||||
| Stainless Steel | Inert | Areas Ratio (Inert/Stainless Steel) | Stainless Steel | Inert | Height Ratio (Inert/Stainless Steel) | |
| Fumonisin B1 | 32578 | 122878 | 3.77 | 399544 | 2415567 | 6.05 |
| Fumonisin B2 | 23427 | 151719 | 6.48 | 383130 | 3869822 | 10.10 |
| Fumonisin B3 | 29864 | 143302 | 4.80 | 472279 | 3444421 | 7.29 |
| Ergocristine | 171197 | 195562 | 1.14 | 3865898 | 4450058 | 1.15 |
| Ergocristinine | 1393116 | 1583053 | 1.14 | 29212317 | 32975663 | 1.13 |
| Ergotamine | 433635 | 493003 | 1.14 | 8149518 | 9274156 | 1.14 |
| Ergotaminine | 397370 | 462119 | 1.16 | 7885403 | 9237991 | 1.17 |
| Ergocryptine | 446481 | 522204 | 1.17 | 9671753 | 11360839 | 1.17 |
| Ergocryptinine | 658788 | 778972 | 1.18 | 13680420 | 16765348 | 1.23 |
| Ergocornine | 370509 | 387025 | 1.04 | 7248981 | 7732744 | 1.07 |
| Ergocorninine | 590167 | 704029 | 1.19 | 12052359 | 14389283 | 1.19 |
| Ergosine | 445243 | 486620 | 1.09 | 8630932 | 9366602 | 1.09 |
| Ergosinine | 439026 | 496734 | 1.13 | 7820785 | 8740527 | 1.12 |
| T-2 | 43286 | 56535 | 1.31 | 1046233 | 1394735 | 1.33 |
| HT-2 | 10183 | 15221 | 1.49 | 216703 | 323765 | 1.49 |
| Tentoxin | 70973 | 95175 | 1.34 | 1577164 | 2131907 | 1.35 |
| Ochratoxin | 173686 | 190060 | 1.09 | 4039682 | 4411953 | 1.09 |
| Diacetoxyscirpenol | 47850 | 68139 | 1.42 | 846403 | 1208826 | 1.43 |
| Fusarenone X | 3865 | 7668 | 1.98 | 60409 | 121790 | 2.02 |
| 15-acetyl-DON | 17055 | 31369 | 1.84 | 269862 | 517570 | 1.92 |
| 3-acetyldeoxyvinalenol | 13353 | 22613 | 1.69 | 179204 | 296396 | 1.65 |
| Aflatoxin G2 | 171597 | 262824 | 1.53 | 3429501 | 5274354 | 1.54 |
| Aflatoxin G1 | 224058 | 304389 | 1.36 | 4607959 | 6102959 | 1.32 |
| ZON | 25617 | 37162 | 1.45 | 656915 | 927455 | 1.41 |
| Aflatoxin B2 | 159389 | 295648 | 1.85 | 3462489 | 5724754 | 1.65 |
| Aflatoxin B1 | 265935 | 223520 | 0.84 | 5335576 | 4425821 | 0.83 |
| Alpha-zearalenol | 16202 | 30224 | 1.87 | 382092 | 702420 | 1.84 |
| Deoxynivalenol | 6935 | 17346 | 2.50 | 117927 | 281906 | 2.39 |
| Nivalenol | 1790 | 4182 | 2.34 | 25276 | 64495 | 2.55 |
| Altenuene | 63224 | 113850 | 1.80 | 1187958 | 2059700 | 1.73 |
| Alternariol monomethyl ether | 19537 | 31024 | 1.59 | 428922 | 640689 | 1.49 |
| Alternariol | 48204 | 73272 | 1.52 | 837410 | 1302192 | 1.56 |
| Citrinin | 499900 | 1007880 | 2.02 | 5031182 | 9828890 | 1.95 |
| Tenuazonic acid | 21503 | 47828 | 2.22 | 89293 | 197658 | 2.21 |
Methylmalonic Acid
The analysis of methylmalonic acid is an important test for clinical laboratories in identifying a Vitamin B12 deficiency. When utilizing our inert hardware in testing for methylmalonic acid, a 40% improvement in sensitivity was achieved compared to regular hardware. A significant increase in methylmalonic acid peak area and peak height is shown for the inert hardware in Figure 7, below.
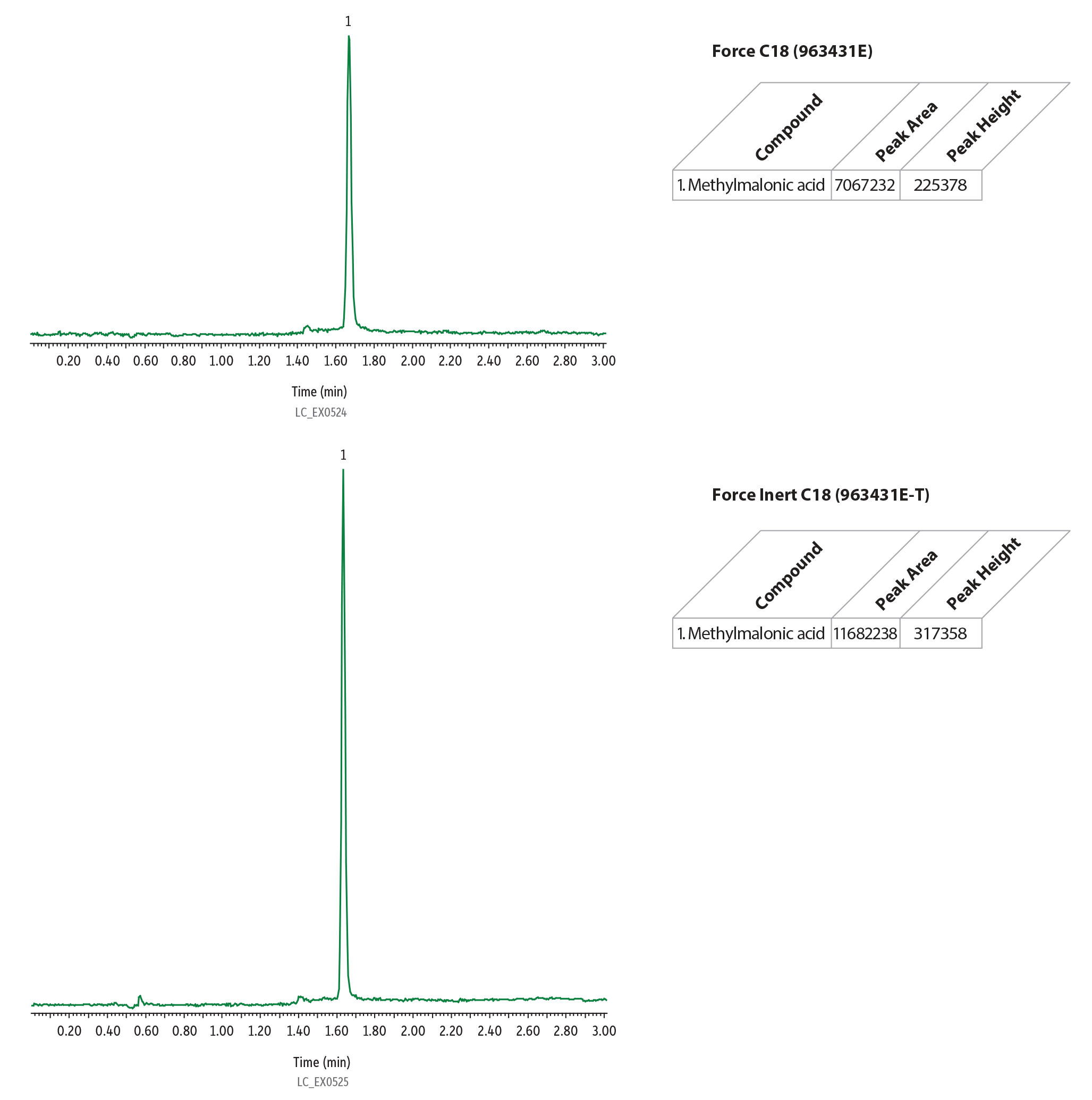
LC_EX0524
Peaks
| Peaks | Precursor Ion | Product Ion | |
|---|---|---|---|
| 1. | MMA | 116.97 | 72.97 |
Conditions
| Column | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp.: | 35 °C | ||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||
| Diluent: | 50:50 Water:methanol | ||||||||||||||||||||||||
| Conc.: | 100 ng/mL | ||||||||||||||||||||||||
| Inj. Vol.: | 5 µL | ||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||
| A: | Water, 0.5% formic acid | ||||||||||||||||||||||||
| B: | Methanol, 0.5% formic acid | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Max Pressure: | 275 bar |
| Detector | Waters Xevo TQ Absolute |
|---|---|
| Ion Source: | Waters Zspray ESI |
| Ion Mode: | ESI- |
| Mode: | MRM |
| Instrument | Waters ACQUITY Premier |
| Notes | Columns are: • Force C18 (cat.# 963431E) • Force Inert C18 (cat.# 963431E-T) |
Veterinary Drugs
The identification of veterinary drugs in food samples, particularly in meats, fish, and eggs, is an important test for ensuring safe food products according to the guidelines outlined by the FDA and the EU. In the analysis of 10 common vet drugs, shown in Figure 8 below, dramatic increases in analyte responses were identified when utilizing the inert column compared to traditional, stainless-steel column.
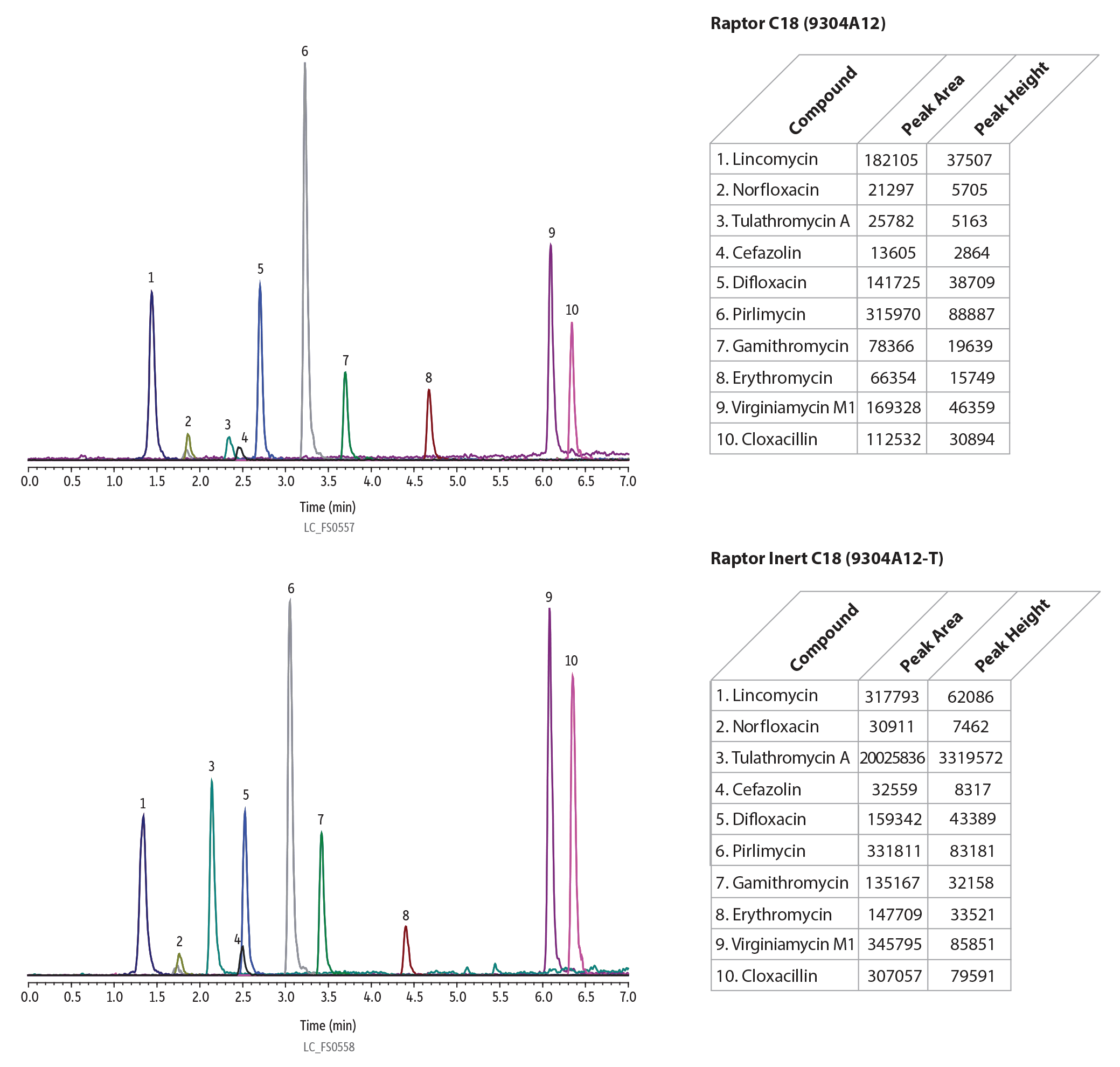
LC_FS0557
Peaks
| Peaks | Precursor Ion | Product Ion | |
|---|---|---|---|
| 1. | Lincomycin | 407.00 | 359.00 |
| 2. | Norfloxacin | 320.00 | 276.00 |
| 3. | Tulathromycin A | 806.60 | 577.00 |
| 4. | Cefazolin | 455.00 | 323.00 |
| 5. | Difloxacin | 400.00 | 356.00 |
| 6. | Pirlimycin | 411.00 | 363.00 |
| 7. | Gamithromycin | 777.00 | 619.00 |
| 8. | Erythromycin | 734.00 | 576.00 |
| 9. | Virginiamycin M1 | 526.00 | 508.00 |
| 10. | Cloxacillin | 436.00 | 277.05 |
Conditions
| Column | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp.: | 35 °C | ||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||
| Diluent: | 50:50 Methanol:water | ||||||||||||||||||||||||
| Conc.: | 10 ng/mL | ||||||||||||||||||||||||
| Inj. Vol.: | 2 µL | ||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||
| A: | Water, 0.1% formic acid | ||||||||||||||||||||||||
| B: | Acetonitrile, 0.1% formic acid | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Max Pressure: | 283 bar |
| Detector | Shimadzu 8060 MS/MS |
|---|---|
| Ion Source: | Electrospray |
| Ion Mode: | ESI+ |
| Mode: | MRM |
| Instrument | Shimadzu Nexera X2 |
| Notes | Columns are: • Raptor C18 (cat.# (9304A12) • Raptor Inert C18 (cat.# (9304A12-T) |
Enhance Performance and Protection with Inert Guards
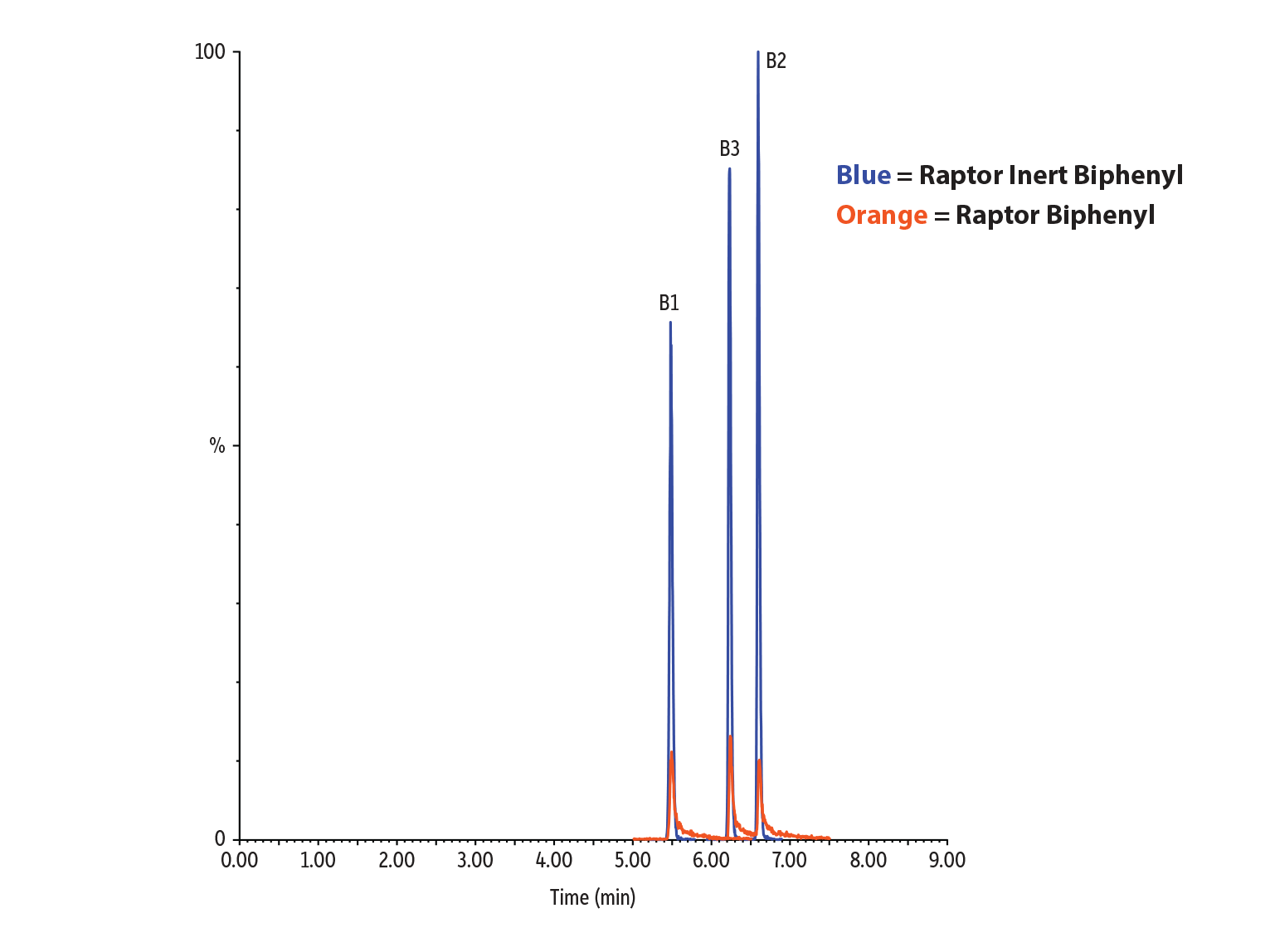
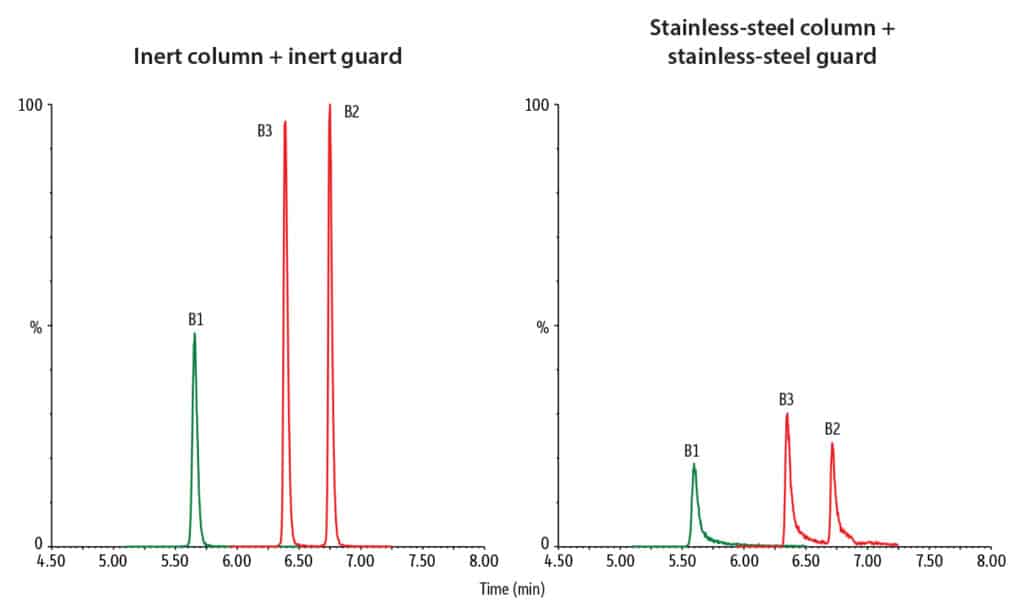
Our inert guard columns not only have the important job of providing protection to your LC system, they also boast improved performance in the analysis of metal-sensitive compounds. In this mycotoxin analysis, we assessed the improvements in analyte recovery and peak shape when utilizing an inert LC column and guard compared to a conventional stainless-steel column and guard. Figure 9 shows the relative peak height increase when switching from a stainless-steel column and guard to an inert column and guard, with increased recoveries in almost all compounds, most notably in the critical fumonisin B1, B2, and B3 compounds. Exceptional improvements in peak shape were also achieved when switching to inert hardware; Figure 10 shows the sharped peaks of fumonisin B1, B2, and B3 with inert hardware.

* (Peak height using inert column and inert guard)/(Peak height using stainless-steel column and stainless-steel guard) x 100%
Exceptional Inertness Meets Exceptional Stationary Phases
We’re introducing our inert LC column technology on three column types: Raptor Biphenyl, Raptor ARC-18, and Force Biphenyl. These new columns help bring the benefits of inert column technology to labs specializing in small molecule LC-MS/MS workflows.
Raptor LC Columns

Raptor LC columns combine the speed of 2.7 µm SPP with the resolution of Ultra Selective Liquid Chromatography (USLC) technology, improving separations and speeding up analysis times with standard HPLC instruments. When speed is your goal, Restek recommends the Raptor line of LC columns.
Learn more at www.restek.com/Raptor
Raptor Inert Biphenyl
This industry-leading Biphenyl is our most popular LC stationary phase. It is particularly adept at separating compounds that are hard to resolve or that elute early on C18 and other phenyl chemistries.
Raptor Inert C18
This traditional end-capped C18 offers the highest hydrophobic retention of any Raptor phase, and it is compatible with a wide range of mobile phases from moderately acidic to neutral (pH 2–8). Whether for food safety or environmental or bioanalytical analyses, this phase offers consistently excellent data quality in less time across myriad reversed-phase applications, matrices, and compound classes.
Raptor Inert ARC-18
The Raptor ARC-18 column features a well-balanced retention profile without the drawbacks of using an ordinary C18 in the harsh, acidic mobile phases needed for mass spectrometry. Even after extended use in these low-pH (≤ 2.0) conditions, the sterically protected ARC-18 offers consistent retention, peak shape, and response for charged bases, neutral acids, small polar compounds, and more.
Force LC Columns

Force fully porous particle (FPP) LC columns are designed and manufactured to handle high-pressure, high-stress conditions. They’re long-lasting, reproducible, and premium quality—backed by our 100% Pure Satisfaction guarantee. When you need greater retention and sharper peaks, Force LC columns are ready to be put to work.
Learn more at www.restek.com/Force
Force Inert Biphenyl
The Force Inert Biphenyl column separates compounds that other phenyl and C18 chemistries can’t. They allow the use of simple, MS-friendly mobile phases and are ideal for when you need to increase retention of hydrophilic aromatics.
Force Inert C18
The general-purpose Restek Force Inert C18 is a conventional monomeric octadecylsilane column suitable for analyses of a wide range of compounds from acidic through slightly basic.
Exceptional Inertness for Your Analysis
As chromatographers, we understand the importance of having confidence in your data. Whether you’re analyzing pesticides, mycotoxins, or any analysis containing metal-sensitive compounds, these new LC columns provide the accuracy, throughput, and performance your lab needs.
Contact your local Restek representative today at www.restek.com/contact-us to pair the benefits of our inert technology with your analysis.