Abstract
The analysis of drugs of abuse in urine can be complicated by matrix components, drug metabolites, and isobaric compounds. In the method developed here, 70 drugs of abuse, including novel psychoactive substances, were analyzed in urine following a simple enzyme hydrolysis sample preparation step. The fast, 8-minute LC-MS/MS method separated all isobars and generated accurate quantitative results at trace levels for all compounds.
Introduction
Testing for drugs of abuse is a necessary task, whether it be for postmortem toxicology, pain management, workplace testing, or a host of other reasons. A variety of biological specimens can be used, but urine is often preferred because it is relatively easy to collect, sample volumes typically exceed what is needed, and target analyte concentrations tend to be higher than in other matrices [1]. In addition, detection windows in urine are typically 1–7 days for most drugs of abuse and can be even longer in samples from chronic users [2]. For these reasons, urine has been used for decades to test for drugs of abuse.
While urine is a common matrix for drugs of abuse analysis, salt concentrations and matrix effects can present analytical challenges. Dilute-and-shoot methods are one approach, but low-level detection can be difficult to achieve because target analytes are diluted along with matrix components. In addition to matrix issues, drugs of abuse analysis can be complicated by the transformation of some drug compounds into glucuronide metabolites. Through this metabolic pathway, which occurs primarily in the liver, glucuronic acid binds to the parent drug, which increases its solubility in water and enables more efficient urinary excretion [3]. The glucuronide forms can be analyzed directly, but because it can be difficult and expensive to source glucuronide standards, LC-MS/MS methods that target the parent drug instead of the glucuronide forms may be advantageous, although effective chromatographic separation is essential when isobars are present.
In order to measure parent drugs directly, a hydrolysis step is required prior to analysis to release glucuronic acid from the drugs of interest [3]. One way of achieving this is through enzymatic hydrolysis using β-glucuronidase, and an example of this reaction is shown in Figure 1. The method developed here employs a simple enzyme hydrolysis sample preparation using β-glucuronidase and LC-MS/MS analysis on a Raptor Biphenyl column in order to accurately quantify 70 drugs of abuse, including isobars, at trace levels in urine.
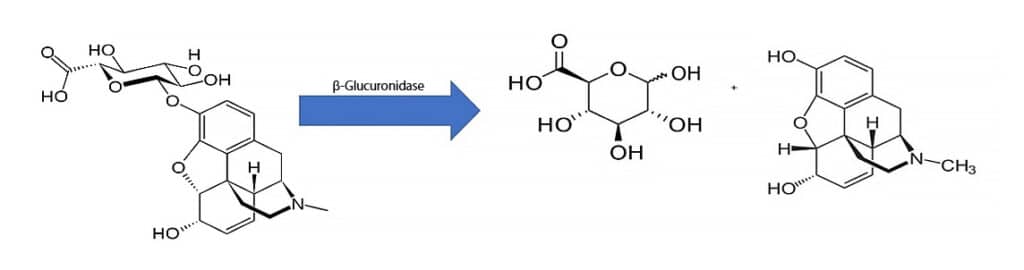
When performing enzyme hydrolysis, it is best practice to use a hydrolysis control, but there are some things to consider first. A hydrolysis control is a urine sample that has been fortified at a known concentration with a glucuronide standard, and its purpose is to demonstrate that the hydrolysis reaction is complete. Because it is more difficult to fully hydrolyze a sample when the analytes are present at high concentrations, it is important that the hydrolysis control be fortified at the higher end of the linearity range [4]. Another consideration when using a hydrolysis control is making sure that the fortified concentration is calculated and prepared correctly. This means taking into account the molecular weight of the glucuronide form in addition to the molecular weight of the parent drug. For example, the molecular weight of morphine is 285.3 g/mol, and the molecular weight of morphine-3-β-glucuronide is 461.4 g/mol. If the molecular weight of the glucuronide form is not considered, then the actual concentration of parent drug in the hydrolysis control will be lower than the target concentration. Accurate fortification levels can be calculated using Formula 1.

Experimental
Master Mix
The following sample preparation uses a master mix containing IMCSzyme RT genetically modified β-glucuronidase (IMCS Irmo, SC). The basic ratio for this master mix is 4 µL of IMCS RT enzyme, 8 µL of IMCS RT buffer, 4.7 µL of water, and 3.3 µL of internal standard (Table I). The total volume of master mix that is prepared should be adjusted to accommodate the number of samples in a batch as each sample is spiked with 20 µL of master mix.
Table I: Internal Standard Concentrations in Urine
| Internal Standard | Concentration ng/mL |
| Norbuprenorphine-D4 | 50 |
| Fentanyl-D5 | 50 |
| Buprenorphine-D4 | 50 |
| 6-Acetylmorphine-D3 | 100 |
| Norfentanyl-D5 | 100 |
| LSD-D3 | 100 |
| PCP-D5 | 250 |
| THC-COOH-D9 | 250 |
| EDDP-D3 | 400 |
| Paroxetine-D6 | 400 |
| Amitriptyline-D3 | 400 |
| Methadone-D3 | 400 |
| Oxazepam-D5 | 400 |
| Alpha-hydroxyalprazolam-D5 | 400 |
| Nordiazepam-D5 | 400 |
| Temazepam-D5 | 400 |
| Morphine-D3 | 400 |
| Oxymorphone-D3 | 400 |
| Hydromorphone-D3 | 400 |
| MDMA-D5 | 400 |
| Haloperidol-D4 | 400 |
| Oxycodone-D6 | 400 |
| Tramadol-13C-D3 | 400 |
| Ketamine-D4 | 400 |
| 7-Aminoclonazepam-D4 | 400 |
| Methamphetamine-D5 | 750 |
| Phenobarbital-D9 | 750 |
Calibrators, Quality Control Samples, and Urine Sample Preparation
Control urine or sample (20 µL) was added to a 1.5 mL microcentrifuge tube along with 20 µL of the premade master mix. Samples were vortexed for 10 seconds and left to incubate at room temperature for 20 minutes. After incubation, 260 µL of diluent (90:10 0.1% formic acid in water:0.1% formic acid in methanol) was added to each sample. The samples were vortexed for 10 seconds and centrifuged for 10 minutes at 3700 rpm. A 100 µL aliquot of supernatant was added to a 200 µL vial insert, and the samples were moved to the LC-MS/MS for analysis. Fortified calibration standards and quality control samples were prepared at the concentrations shown in Table II.
Table II: Analytical Ranges for Drugs of Abuse in Urine
| Analyte | Analytical Range (ng/mL) | QC Range (ng/mL) | ||||||||||
| Cal H | Cal G | Cal F | Cal E | Cal D | Cal C | Cal B | Cal A | QC LOQ | QC Low | QC Med | QC High | |
| 6-β-Naltrexol | 2 | 5 | 20 | 30 | 40 | 60 | 80 | 100 | 5 | 15 | 35 | 75 |
| Acetyl fentanyl | ||||||||||||
| Buprenorphine | ||||||||||||
| Fentanyl | ||||||||||||
| Sufentanil | ||||||||||||
| 6-Monoacetylmorphine | 4 | 10 | 40 | 60 | 80 | 120 | 160 | 200 | 10 | 30 | 70 | 150 |
| 7-Hydroxymitragynine | ||||||||||||
| LSD | ||||||||||||
| Norbuprenorphine | ||||||||||||
| Norfentanyl | ||||||||||||
| Naloxone | 8 | 20 | 80 | 120 | 160 | 240 | 320 | 400 | 20 | 60 | 140 | 300 |
| Benzoylecgonine | 10 | 25 | 100 | 150 | 200 | 300 | 400 | 500 | 25 | 75 | 175 | 375 |
| PCP | ||||||||||||
| THC-COOH (delta-9-COOH) | ||||||||||||
| 7-Aminoclonazepam | 20 | 50 | 200 | 300 | 400 | 600 | 800 | 1000 | 50 | 150 | 350 | 750 |
| 9-Hydroxyrisperidone | ||||||||||||
| Alpha-OH-alprazolam | ||||||||||||
| Amitriptyline | ||||||||||||
| Amphetamine | ||||||||||||
| Carisoprodol | ||||||||||||
| Citalopram | ||||||||||||
| Codeine | ||||||||||||
| Cyclobenzaprine | ||||||||||||
| Dehydroaripiprazole | ||||||||||||
| Desmethyldoxepin | ||||||||||||
| Dextromethorphan | ||||||||||||
| Duloxetine | ||||||||||||
| EDDP | ||||||||||||
| Haloperidol | ||||||||||||
| Hydrocodone | ||||||||||||
| Hydromorphone | ||||||||||||
| Hydroxybupropion | ||||||||||||
| Lamotrigine | ||||||||||||
| Lorazepam | ||||||||||||
| MDMA | ||||||||||||
| Meprobamate | ||||||||||||
| Methadone | ||||||||||||
| Mirtazapine | ||||||||||||
| Morphine | ||||||||||||
| Naltrexone | ||||||||||||
| N-Desmethyltapentadol | ||||||||||||
| Nordiazepam | ||||||||||||
| Norfluoxetine | ||||||||||||
| Norhydrocodone | ||||||||||||
| Norketamine | ||||||||||||
| Normeperidine | ||||||||||||
| Noroxycodone | ||||||||||||
| Nortriptyline | ||||||||||||
| O-Desmethyltramadol | ||||||||||||
| O-Desmethyl-venlafaxine | ||||||||||||
| Oxazepam | ||||||||||||
| Oxycodone | ||||||||||||
| Oxymorphone | ||||||||||||
| Paroxetine | ||||||||||||
| 7-Hydroxyquetiapine | ||||||||||||
| Ritalinic acid | ||||||||||||
| Norsertraline | ||||||||||||
| Temazepam | ||||||||||||
| Tramadol | ||||||||||||
| Trazodone | ||||||||||||
| Venlafaxine | ||||||||||||
| Xylazine | ||||||||||||
| Zolpidem phenyl-4-carboxylic acid | ||||||||||||
| Butalbital | 40 | 100 | 400 | 600 | 800 | 1200 | 1600 | 2000 | 100 | 300 | 700 | 1500 |
| Cotinine | ||||||||||||
| Methamphetamine | ||||||||||||
| Phenobarbital | ||||||||||||
| Phentermine | ||||||||||||
| Gabapentin | 100 | 250 | 1000 | 1500 | 2000 | 3000 | 4000 | 5000 | 250 | 750 | 1750 | 3750 |
| Pregabalin | ||||||||||||
Hydrolysis Control
The hydrolysis control prepared in this method contained four analytes fortified to equal 1000 ng/mL of parent drug when liberated. Morphine-3-β-D-glucuronide; hydromorphone-3-β-D-glucuronide; amitriptyline-N-β-D-glucuronide; and oxazepam glucuronide were spiked into urine using Formula 1. After fortification, 20 µL of the hydrolysis control was added to a 1.5 mL microcentrifuge tube along with 20 µL of the premade master mix. Samples were vortexed for 10 seconds and left to incubate at room temperature for 20 minutes. After incubation, 260 µL of diluent (90:10 0.1% formic acid in water:0.1% formic acid in methanol) was added to each sample. The samples were vortexed for 10 seconds and centrifuged for 10 minutes at 3700 rpm. A 100 µL aliquot of supernatant was added to a 200 µL vial insert, and the samples were moved to the LC-MS/MS for analysis.
Instrument Conditions
| Analytical column: | Raptor Biphenyl 2.7 µm, 50 mm x 2.1 mm (cat.# 9309A52) | |
| Guard column: | Raptor Biphenyl EXP guard column cartridge 5 x 2.1 mm, 2.7 µm (cat.# 9309A0252) | |
| Mobile phase A: | 0.1% Formic acid in water | |
| Mobile phase B: | 0.1% Formic acid in methanol | |
| Gradient | Time (min) | %B |
| 0.00 | 10 | |
| 6.00 | 75 | |
| 7.00 | 100 | |
| 7.01 | 10 | |
| 8.00 | 10 | |
| Flow rate: | 0.6 mL/min | |
| Injection volume: | 2 µL | |
| Column temp.: | 45 °C | |
| Ion mode: | Positive and negative ESI | |
Results and Discussion
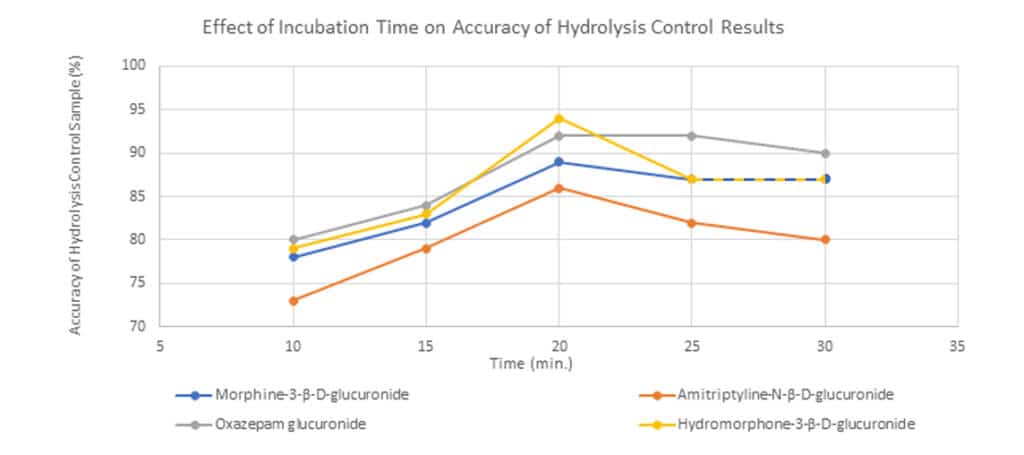
Optimization of Incubation Time
Different incubation times were tested to determine the optimal time for IMCSzyme RT to perform maximum hydrolysis. This test was performed using a hydrolysis control sample, and incubation times of 10, 15, 20, 25, and 30 minutes were evaluated. An incubation time of 20 minutes produced results closest to the nominal value for all four compounds (Figure 2).
Optimization of Diluent Volume
In order to reduce the amount of matrix injected on the column and still achieve the required sensitivity for the method, different diluent volumes (260 µL, 360 µL, 460 µL, and 560 µL) were examined. Overall, 260 µL of diluent was chosen as the best compromise when considering matrix loading, peak shapes, and sensitivity.
Evaluation of Hydrolysis Efficiency
To assess hydrolysis efficiency, three hydrolysis controls were prepared and analyzed over the course of three days (n=9). The control samples showed acceptable results with all analytes falling within ±15% of the expected value for both intraday and interday repeatability studies (Table III). Precision results also passed with %RSD values of <10%.
Table III: Interday Repeatability of the Hydrolysis Control (Average Across Three-Day Study)
| Morphine | ||||
| Sample | Expected (ng/mL) | Average (ng/mL) | % Difference | % RSD |
| 1 | 1000 | 1110 | 11.0 | 7.6 |
| 2 | 1000 | 915 | 8.5 | 9.3 |
| 3 | 1000 | 950 | 5.0 | 8.9 |
| Hydromorphone | ||||
| Sample | Expected (ng/mL) | Average (ng/mL) | % Difference | % RSD |
| 1 | 1000 | 1040 | 4.0 | 2.5 |
| 2 | 1000 | 1100 | 10.0 | 2.4 |
| 3 | 1000 | 1090 | 9.0 | 2.4 |
| Amitriptyline | ||||
| Sample | Expected (ng/mL) | Average (ng/mL) | % Difference | % RSD |
| 1 | 1000 | 1150 | 15.0 | 6.9 |
| 2 | 1000 | 962 | 3.8 | 8.3 |
| 3 | 1000 | 1010 | 1.0 | 0.0 |
| Oxazepam | ||||
| Sample | Expected (ng/mL) | Average (ng/mL) | % Difference | % RSD |
| 1 | 1000 | 977 | 2.3 | 4.1 |
| 2 | 1000 | 940 | 6.0 | 4.2 |
| 3 | 1000 | 880 | 12.0 | 0.0 |
Chromatographic Performance
The analysis and separation of 70 drugs of abuse in urine was achieved in a fast, 8-minute cycle time on a Raptor Biphenyl 50 x 2.1 mm, 2.7 µm column by LC-MS/MS as demonstrated in Figure 3.
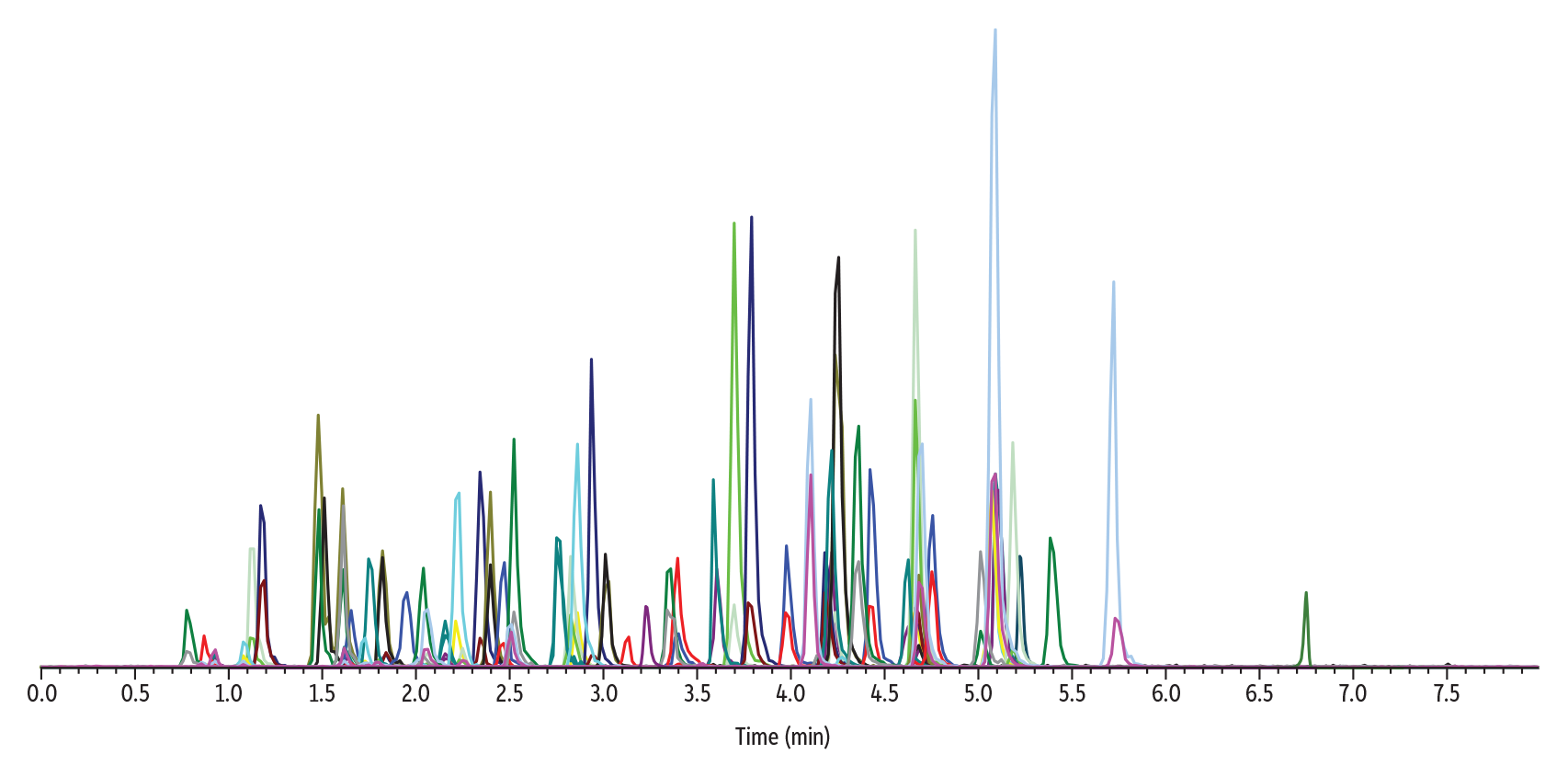
LC_CF0806
Peaks
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion 1 | Product Ion 2 | Polarity | |
|---|---|---|---|---|---|---|---|
| 1. | Cotinine | 0.78 | 800 | 177.1 | 80.0 | 98.1 | + |
| 2. | Morphine | 0.85 | 400 | 286.2 | 152.1 | 165.0 | + |
| 3. | Pregabalin | 0.88 | 2000 | 160.2 | 142.1 | 55.0 | + |
| 4. | Oxymorphone | 0.92 | 400 | 302.1 | 227.2 | 198.2 | + |
| 5. | Hydromorphone | 1.08 | 400 | 286.2 | 184.9 | 156.9 | + |
| 6. | Amphetamine | 1.13 | 400 | 136.2 | 91.0 | 65.1 | + |
| 7. | Gabapentin | 1.18 | 2000 | 172.2 | 154.0 | 137.1 | + |
| 8. | Methamphetamine | 1.48 | 800 | 150.2 | 91.1 | 119.0 | + |
| 9. | Phentermine | 1.62 | 800 | 150.2 | 91.1 | 133.1 | + |
| 10. | Noroxycodone | 1.62 | 400 | 302.1 | 227.0 | 197.9 | + |
| 11. | Naloxone | 1.65 | 160 | 328.3 | 310.1 | 212.3 | + |
| 12. | Norhydrocodone | 1.73 | 400 | 286.1 | 199.0 | 128.2 | + |
| 13. | <a class="cmpd_link" title="View compound information for O-Desmethyltramadol” title=”View compound information for O-Desmethyltramadol” href=”https://ez.restek.com/compound/view/en/80456-81-1/O-Desmethyltramadol”>O-Desmethyltramadol | 1.75 | 400 | 250.1 | 58.0 | 42.0 | + |
| 14. | Codeine | 1.80 | 400 | 300.2 | 152.0 | 165.1 | + |
| 15. | MDMA | 1.82 | 400 | 194.1 | 163.0 | 135.1 | + |
| 16. | 6-Acetylmorphine | 1.84 | 80 | 328.2 | 211.0 | 165.0 | + |
| 17. | Oxycodone | 1.95 | 400 | 316.2 | 298.0 | 169.0 | + |
| 18. | Naltrexone | 2.04 | 400 | 342.2 | 324.0 | 267.0 | + |
| 19. | Hydrocodone | 2.06 | 400 | 300.2 | 199.0 | 128.0 | + |
| 20. | <a class="cmpd_link" title="View compound information for O-desmethylvenlafaxine” title=”View compound information for O-desmethylvenlafaxine” href=”https://ez.restek.com/compound/view/en/93413-62-8/O-desmethylvenlafaxine”>O-desmethylvenlafaxine | 2.16 | 400 | 164.1 | 58.1 | 107.0 | + |
| 21. | 6-β-Naltrexol | 2.21 | 40 | 344.2 | 326.1 | 308.1 | + |
| 22. | Lamotrigine | 2.24 | 400 | 255.9 | 211.1 | 145.0 | + |
| 23. | Ritalinic acid | 2.34 | 400 | 220.1 | 84.1 | 56.2 | + |
| 24. | N-Desmethyltapentadol | 2.40 | 400 | 208.1 | 121.2 | 107.1 | + |
| 25. | Norketamine | 2.46 | 400 | 224.1 | 125.0 | 89.1 | + |
| 26. | Hydroxybupropion | 2.50 | 400 | 256.0 | 130.2 | 166.0 | + |
| 27. | Norfentanyl | 2.53 | 80 | 233.1 | 84.1 | 55.0 | + |
| 28. | 7-Hydroxyquetiapine | 2.71 | 400 | 400.2 | 269.0 | 208.0 | + |
| 29. | Tramadol | 2.76 | 400 | 264.2 | 58.0 | 77.1 | + |
| 30. | Xylazine | 2.84 | 400 | 221.9 | 90.1 | 71.9 | + |
| 31. | Zolpidem Phenyl-4-carboxylic acid | 2.84 | 400 | 338.1 | 265.1 | 219.0 | + |
| 32. | Benzoylecgonine | 2.85 | 200 | 290.1 | 168.1 | 77.1 | + |
| 33. | Normeperidine | 2.94 | 400 | 234.1 | 160.2 | 91.0 | + |
| 34. | Meprobamate | 3.01 | 400 | 219.1 | 158.2 | 97.0 | + |
| 35. | 7-Aminoclonazepam | 3.07 | 400 | 286.1 | 121.2 | 250.1 | + |
| 36. | Phenobarbital | 3.23 | 800 | 230.8 | 187.8 | 85.0 | – |
| 37. | Venlafaxine | 3.35 | 400 | 278.4 | 260.4 | 195.1 | + |
| 38. | Mirtazapine | 3.39 | 400 | 266.1 | 72.1 | 195.1 | + |
| 39. | Butalbital | 3.47 | 800 | 222.9 | 180.0 | 84.9 | – |
| 40. | Norbuprenorphine | 3.51 | 80 | 414.3 | 152.2 | 165.2 | + |
| 41. | LSD | 3.61 | 80 | 324.2 | 223.1 | 208.0 | + |
| 42. | 7-Hydroxymitragynine | 3.65 | 80 | 415.5 | 190.1 | 174.9 | + |
| 43. | 9-Hydroxyrisperidone | 3.77 | 400 | 427.2 | 110.2 | 207.1 | + |
| 44. | Acetyl fentanyl | 3.79 | 40 | 323.2 | 188.0 | 105.0 | + |
| 45. | Citalopram | 3.94 | 400 | 325.1 | 109.1 | 262.0 | + |
| 46. | Desmethyldoxepin | 3.99 | 400 | 266.1 | 107.1 | 115.0 | + |
| 47. | Trazodone | 4.10 | 400 | 372.3 | 148.0 | 260.4 | + |
| 48. | Dextromethorphan | 4.18 | 400 | 272.1 | 215.1 | 170.9 | + |
| 49. | Haloperidol | 4.18 | 400 | 377.2 | 170.9 | 123.0 | + |
| 50. | Fentanyl | 4.20 | 40 | 337.2 | 188.0 | 105.1 | + |
| 51. | Norfluoxetine | 4.23 | 400 | 296.3 | 134.3 | 104.9 | + |
| 52. | PCP | 4.25 | 200 | 244.1 | 86.1 | 159.1 | + |
| 53. | Buprenorphine | 4.27 | 40 | 468.3 | 55.1 | 414.2 | + |
| 54. | Carisoprodol | 4.43 | 400 | 261.1 | 176.0 | 62.0 | + |
| 55. | EDDP | 4.64 | 400 | 278.1 | 234.3 | 249.2 | + |
| 56. | Duloxetine | 4.65 | 400 | 298.1 | 154.1 | 188.2 | + |
| 57. | Paroxetine | 4.65 | 400 | 330.1 | 192.2 | 70.1 | + |
| 58. | Nortriptyline | 4.67 | 400 | 264.1 | 91.1 | 115.2 | + |
| 59. | Cyclobenzaprine | 4.67 | 400 | 276.2 | 215.0 | 189.0 | + |
| 60. | Sufentanil | 4.70 | 40 | 387.2 | 238.1 | 111.1 | + |
| 61. | Amitriptyline | 4.82 | 400 | 278.1 | 91.1 | 202.1 | + |
| 62. | Norsertraline HCl | 5.02 | 400 | 292.0 | 275.0 | 159.0 | + |
| 63. | Lorazepam | 5.02 | 400 | 321.1 | 229.0 | 275.0 | + |
| 64. | Methadone | 5.08 | 400 | 310.2 | 264.9 | 105.1 | + |
| 65. | Oxazepam | 5.08 | 400 | 287.1 | 268.8 | 241.2 | + |
| 66. | Dehydro aripiprazole | 5.18 | 400 | 446.2 | 285.0 | 98.1 | + |
| 67. | alpha-Hydroxyalprazolam | 5.33 | 400 | 325.1 | 297.0 | 216.2 | + |
| 68. | Nordiazepam | 5.40 | 400 | 271.0 | 139.9 | 208.0 | + |
| 69. | Temazepam | 5.71 | 400 | 301.1 | 255.1 | 282.9 | + |
| 70. | THC-COOH | 6.73 | 200 | 343.0 | 298.9 | 244.8 | – |
Conditions
| Column | Raptor Biphenyl (cat.# 9309A52) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 50 mm x 2.1 mm ID | ||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||
| Guard Column: | Raptor Biphenyl EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9309A0252) | ||||||||||||||||||||||||
| Temp.: | 45 °C | ||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||
| Diluent: | 90:10 Water, 0.1% formic acid:methanol, 0.1% formic acid | ||||||||||||||||||||||||
| Inj. Vol.: | 2 µL | ||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||
| A: | Water, 0.1% formic acid | ||||||||||||||||||||||||
| B: | Methanol, 0.1% formic acid | ||||||||||||||||||||||||
|
| Detector | SCIEX Triple Quad 4500 |
|---|---|
| Ion Source: | Electrospray |
| Ion Mode: | ESI+/ESI- |
| Instrument | Shimadzu Nexera X2 |
| Sample Preparation | 20 µL of a calibrator control in urine was added to a 1.5 mL microcentrifuge tube along with 20 µL of a premade enzyme hydrolysis master mix. The sample was vortexed for 10 seconds and left to incubate at room temperature for 20 minutes. After the incubation, 260 µL of the diluent [water, 0.1 % formic acid: methanol, 0.1 % formic acid 90:10 (v:v)] was added. The sample was vortexed for 10 seconds and centrifuged for 10 minutes at 3700 rpm. One hundred microliters was added to a vial insert (cat. #21776) in a 2.0 mL, amber, short-cap vial (cat.# 21142) and capped with a 9 mm short cap (cat.# 24497) and injected on the LC-MS/MS for analysis. |
Resolution of Isobars
Drugs of abuse assays often contain multiple isobars that require chromatographic separation. These analytes must be resolved in order to accurately quantitate each compound because they cannot be distinguished by the MS alone. A target resolution of 1.5 (baseline) or greater is ideal for quantitative work. Resolution can be calculated using Formula 2 where tR is retention time and W is peak width.
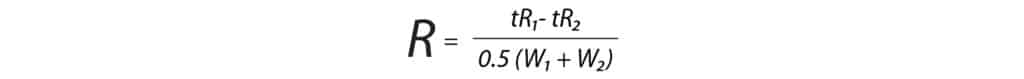
As shown in Table IV, nine groups of isobars were analyzed, and the resolution values within each group were calculated using Formula 2. The Raptor Biphenyl column provided good selectivity and effectively separated the compounds within all nine isobar groups.
Table IV: Isobar Resolution at High QC
| Isobar Group | Name | Molecular Weight (g/mol) | Retention Time (min) | Peak Width | Resolution |
| 1 | Morphine | 285.3 | 0.87 | 0.105 | 1.9 |
| Hydromorphone | 1.11 | 0.150 | |||
| Norhydrocodone | 1.75 | 0.122 | 8.7 | ||
| 7-Aminoclonazepam | 3.07 | 0.180 | |||
| 2 | Oxymorphone | 301.3 | 0.95 | 0.12 | 5.8 |
| Noroxycodone | 1.65 | 0.12 | |||
| 3 | Methamphetamine | 149.2 | 1.41 | 0.15 | 1.2* |
| Phentermine | 1.56 | 0.11 | |||
| 4 | Naloxone | 327.3 | 1.65 | 0.15 | 1.5 |
| 6-Acetylmorphine | 1.84 | 0.1 | |||
| 5 | Codeine | 299.4 | 1.80 | 0.113 | 1.7 |
| Hydrocodone | 2.07 | 0.201 | |||
| 6 | O-desmethylvenlafaxine | 263.4 | 2.11 | 0.15 | 3.3 |
| Tramadol | 2.70 | 0.203 | |||
| Mirtazapine | 3.39 | 0.201 | 7.2 | ||
| Nortriptyline | 4.66 | 0.150 | |||
| 7 | Lamotrigine | 256.1 | 2.24 | 0.150 | 1.6 |
| Hydroxybupropion | 255.7 | 2.50 | 0.185 | ||
| 8 | Citalopram | 324.4 | 3.98 | 0.2 | 6.6 |
| Alpha-hydroxyalprazolam | 5.36 | 0.22 | |||
| 9 | EDDP | 278.1 | 4.64 | 0.11 | 1.5 |
| Amitriptyline | 4.81 | 0.12 |
*If additional resolution of methamphetamine and phentermine is required, a Raptor Biphenyl 50 x 4.6 mm, 2.7 µm column is recommended.
Accuracy and Precision
Precision and accuracy analyses were performed over the course of three days using three sets each day (n=9). Method accuracy was successfully demonstrated with QC LLOQ, QC Low, QC Med, and QC High results falling within ±15% of the expected values for all analytes. The percent relative standard deviation (%RSD) for intraday and interday testing fell below 9.69%, indicating acceptable method precision.
Linearity
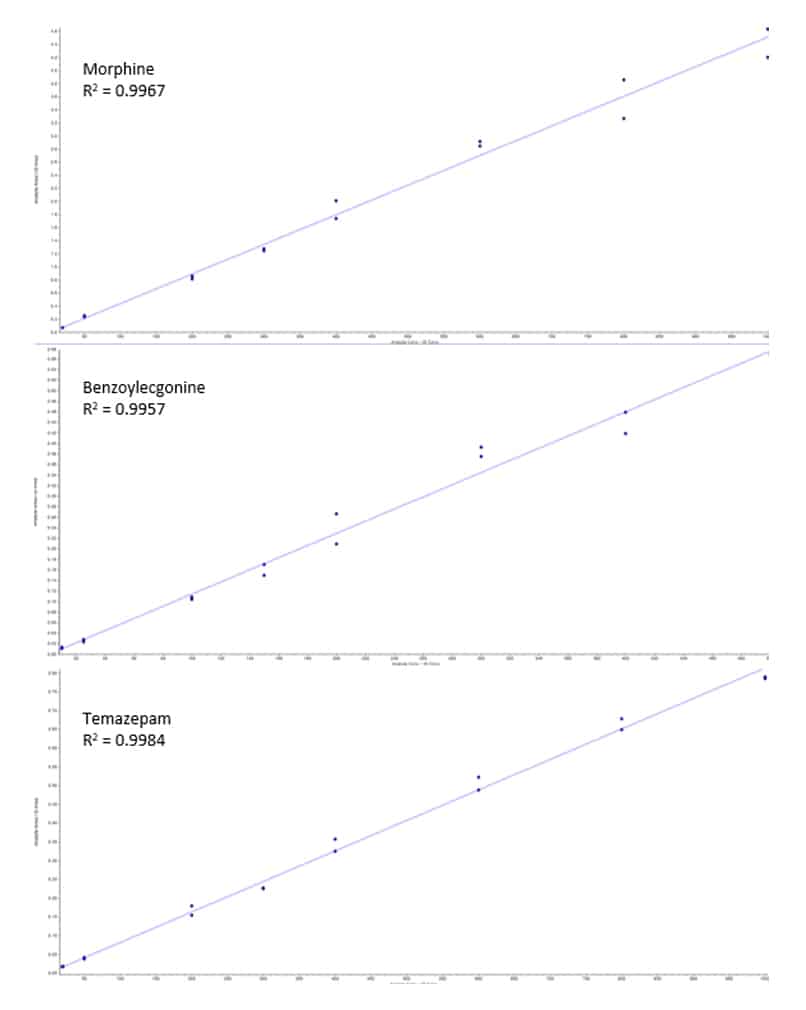
Calibration curves were built using standard over internal standard ratios. Linearity was demonstrated using a 1/x2 weighted linear regression, and all analytes showed acceptable R2 values of 0.991 or greater. The example calibration curves shown in Figure 4 highlight three analytes that represent early, middle, and late eluting compounds: morphine (0.85 min); benzoylecgonine (2.85 min); and temazepam (5.71 min). The linear ranges varied across the different drugs of abuse and are presented above in Table II.
Column Robustness
Because urine is a relatively dirty matrix, using a guard column is recommended to remove matrix components and protect the analytical column from contamination. Column robustness was tested by running more than 250 matrix injections on the same guard column and analytical column. This evaluation showed good results, with the retention times of the first and last injections having a percent difference of 4.52% or less for all analytes with no observed increase in back pressure. This demonstrates that the performance of both the guard and analytical columns is robust over many injections.
Conclusion
The method developed here provides a quick, effective approach for sample preparation and LC-MS/MS analysis of 70 drugs of abuse in urine. Separation of all drugs, including isobars, was achieved with this rapid and reliable 8-minute method, allowing high-throughput, quantitative analysis at trace levels. This method demonstrated successful precision, accuracy, and linearity for all analytes. It also showed that enzymatic hydrolysis was effective in cleaving the glucuronide from the analyte of interest, allowing lower limits of detection and the ability to report total concentrations.
References
- K. E. Moeller, et al., Urine drug screening: Practical guide for clinicians. Mayo Clinic Proceedings. 83, (1) (2008) 66-76. https://www.mayoclinicproceedings.org/article/s0025-6196(11)61120-8/fulltext
- J. Cybulski, Urine analysis: The good, the dad, and the ugly. LCGC International. 12 (2016) (3) 13-16. https://www.chromatographyonline.com/view/urine-analysis-good-bad-and-ugly
- J. Neifeld, Urine hydrolysis: how did I choose which enzyme to use? Blog. Biotage
https://www.biotage.com/blog/urine-hydrolysis-how-did-i-choose-which-enzyme-to-use (Accessed April 17, 2025) - K. Skov, et al., Exploring enzymatic hydrolysis of urine samples for investigation of drugs associated with drug-facilitated sexual assault. Pharmaceuticals. 17 (2023) (21) https://doi.org/10.3390/ph17010013
This method has been developed for research use only; it is not suitable for use in diagnostic procedures without further evaluation.