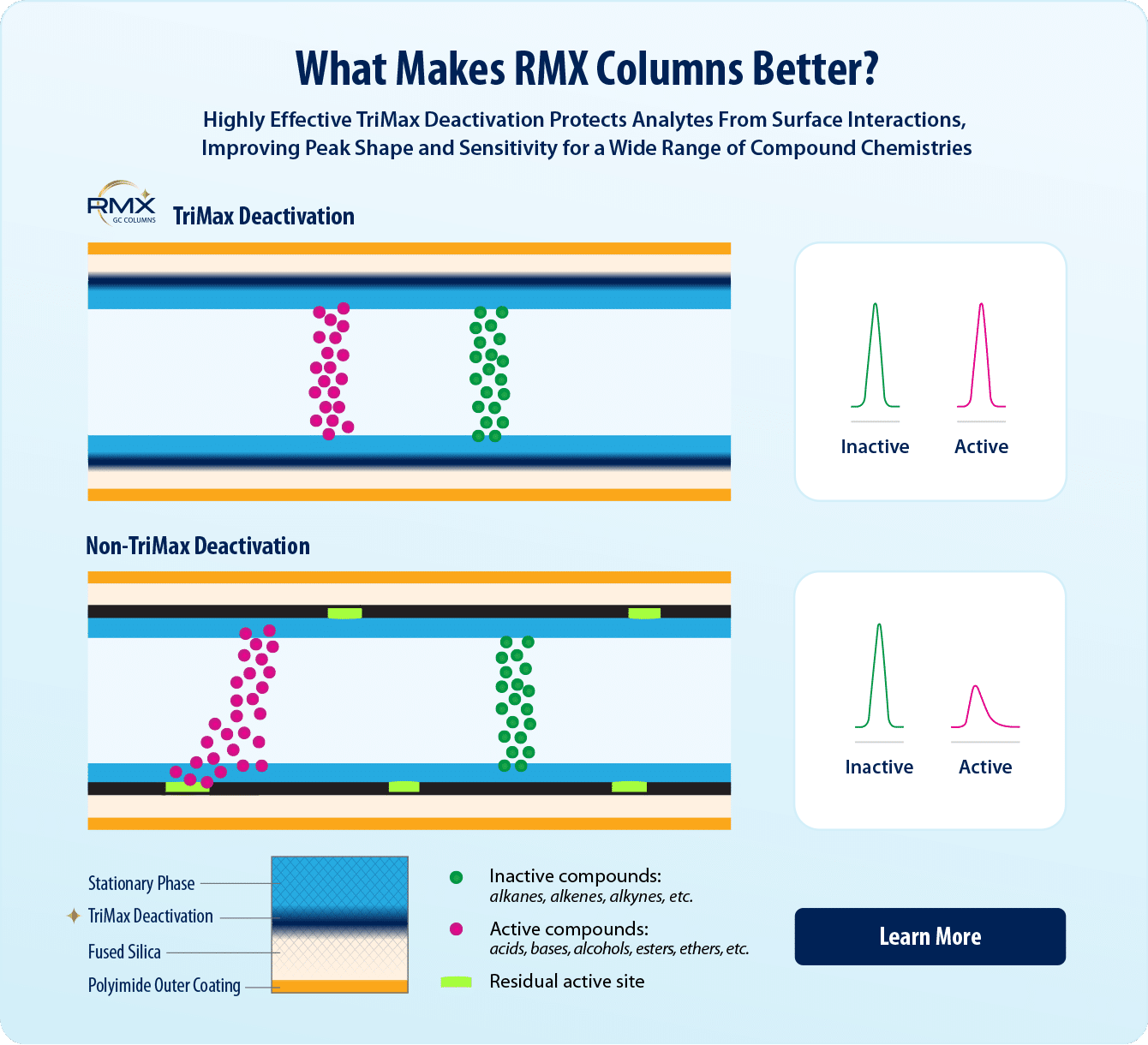
- Next-generation TriMax column deactivation creates a robust neutral surface and exceptionally inert sample flow path.
- Maximum inertness improves peak symmetry for a wide range of challenging drug classes.
- Stable, symmetrical peaks mean more accurate identification for even the most problematic drugs.

Seized drug labs frequently analyze samples via GC-MS to determine if an unknown substance is an illicit drug, and GC column choice plays an integral role in data quality and method reliability. Since a wide variety of chemical compounds need to be identified, midpolarity columns, such as “5 type” columns, are a common choice for drug analysis. Silarylene polymers are structurally modified “5 type” phases that provide enhanced thermal stability, making a “5sil” column the perfect choice for extended lifetime and consistent performance with a mass spectrometer. When analyzing a wide range of drugs, some compound classes exhibit worse behavior than others, which can make drug identification more complex due to tailing peaks and drifting retention times. Acidic compounds (e.g., barbiturates) are prone to uneven phase partitioning due to strong interactions between weakly acidic N-H groups and silanols on the surface of the GC column. Other drugs may have basic functionalities (e.g., amines) and form hydrogen bonds with the surface, again delaying partitioning out of the column phase, or sometimes being completely adsorbed. By preventing surface silanol interactions, seized drug labs can see improved performance for the most problematic compounds across a wide range of functionalities.
To minimize the impact of silanols, Restek has developed a next-generation TriMax deactivation that is applied to all columns in the RMX family. This revolutionary treatment creates a robust, highly inert surface that improves peak shape for a wide range of drug classes. Further, analysts can be confident in column performance because each column is individually QC tested with acidic, basic, and neutral compounds to ensure deactivation effectiveness and process control. This makes RMX-5Sil MS columns—which combine plug-in 5sil polarity, high thermal stability, and maximum surface inertness—ideal for improving seized drug identification (Figure 1). Further, as shown in Figure 2, the RMX-5Sil MS column outperforms a competitor’s premium column when comparing peak shape for several difficult basic analytes (phencyclidine, cyclobenzaprine, codeine) as well as a weakly acidic analyte (alprazolam). Under the same instrument conditions, we can see an improvement in peak shape that can only be attributed to the maximum inertness of the RMX-5Sil MS column surface, which minimizes secondary analyte interactions with silanols that would otherwise cause peak tailing and poor asymmetry.
Figure 1: RMX-5Sil MS columns provide excellent chromatographic results for 34 drugs of abuse analyzed by GC-MS in less than 21 minutes.
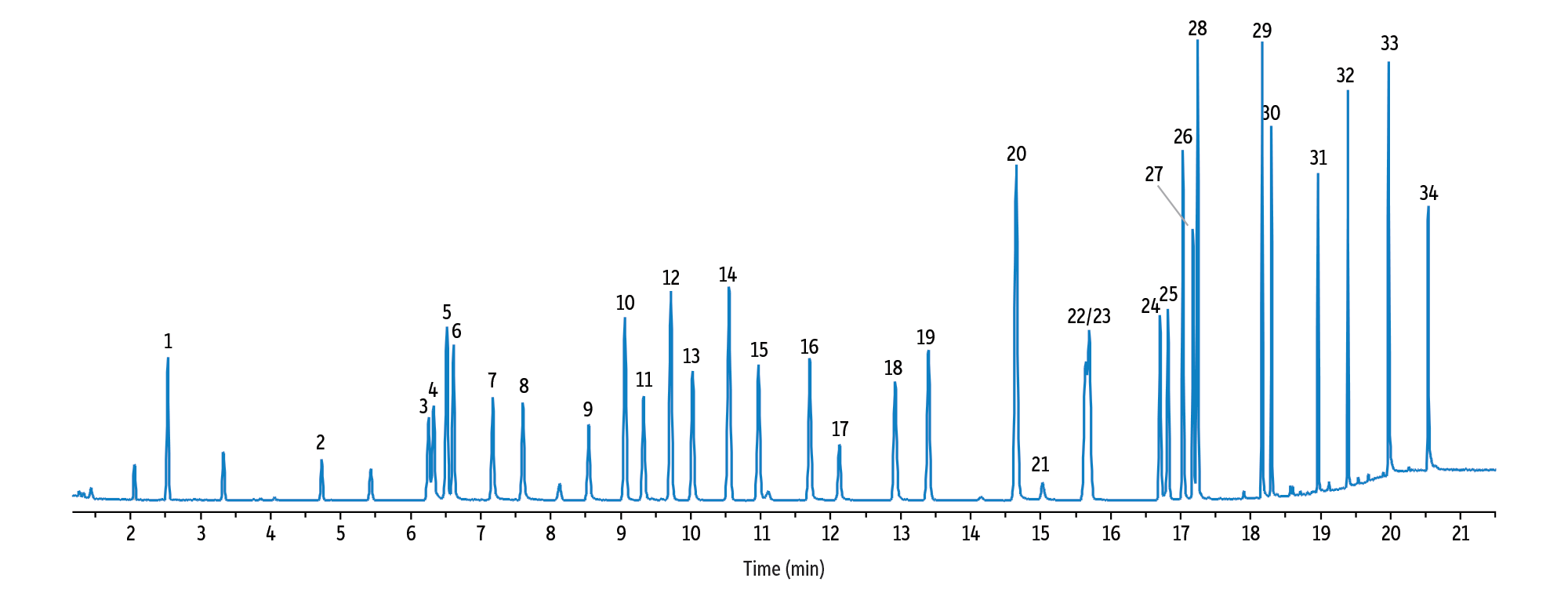
GC_CF1185
Peaks
| Peaks | tR (min) | Conc. (µg/mL) | |
|---|---|---|---|
| 1. | Propofol | 2.53 | 25 |
| 2. | Benzocaine | 4.73 | 25 |
| 3. | Butalbital | 6.25 | 25 |
| 4. | Butabarbital | 6.33 | 25 |
| 5. | Phenacetin | 6.52 | 25 |
| 6. | Cotinine | 6.61 | 25 |
| 7. | Amobarbital | 7.17 | 25 |
| 8. | Pentobarbital | 7.6 | 25 |
| 9. | Secobarbital | 8.54 | 25 |
| 10. | Prilocaine | 9.06 | 25 |
| 11. | Norketamine | 9.33 | 25 |
| Peaks | tR (min) | Conc. (µg/mL) | |
|---|---|---|---|
| 12. | Diphenhydramine | 9.71 | 25 |
| 13. | Ketamine | 10.02 | 25 |
| 14. | Doxylamine | 10.54 | 25 |
| 15. | Phencyclidine (PCP) | 10.97 | 25 |
| 16. | Tramadol | 11.7 | 25 |
| 17. | Phenobarbital | 12.12 | 25 |
| 18. | Chlorpheniramine | 12.92 | 25 |
| 19. | Procaine | 13.39 | 25 |
| 20. | Venlafaxine | 14.65 | 25 |
| 21. | Brompheniramine | 15.02 | 25 |
| 22. | Methadone | 15.64 | 25 |
| Peaks | tR (min) | Conc. (µg/mL) | |
|---|---|---|---|
| 23. | Dextromethorphan | 15.69 | 25 |
| 24. | Amitriptyline | 16.70 | 25 |
| 25. | Cocaine | 16.82 | 25 |
| 26. | Tetracaine | 17.03 | 25 |
| 27. | Imipramine | 17.17 | 25 |
| 28. | Cyclobenzaprine | 17.24 | 25 |
| 29. | Sertraline | 18.16 | 25 |
| 30. | Codeine | 18.29 | 25 |
| 31. | 6-Acetylmorphine | 18.96 | 25 |
| 32. | Heroin | 19.38 | 25 |
| 33. | Zolpidem | 19.97 | 25 |
| 34. | Alprazolam | 20.53 | 25 |
Conditions
| Column | RMX-5Sil MS, 30 m, 0.25 mm ID, 0.25 µm (cat.# 17323) |
|---|---|
| Standard/Sample | |
| Diluent: | Ethyl acetate |
| Conc.: | 25 ppm |
| Injection | |
| Inj. Vol.: | 1 µL split (split ratio 10:1) |
| Liner: | Topaz 4 mm single taper inlet liner with wool (cat.# 23303) |
| Inj. Temp.: | 250 °C |
| Split Vent Flow Rate: | 25 mL/min |
| Oven | |
| Oven Temp.: | 150 °C (hold 1.0 min) to 210 °C at 4 °C/min to 320 °C at 30 °C/min (hold 2.0 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 2.0 mL/min |
| Linear Velocity: | 54 cm/sec @ 150 °C |
| Detector | MS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mode: | Scan | ||||||||
| Scan Program: | |||||||||
| |||||||||
| Transfer Line Temp.: | 280 °C | ||||||||
| Analyzer Type: | Quadrupole | ||||||||
| Source Temp.: | 230 °C | ||||||||
| Electron Energy: | 70 eV | ||||||||
| Tune Type: | PFTBA | ||||||||
| Ionization Mode: | EI | ||||||||
| Instrument | Agilent 7890A GC & 5975C MSD | ||||||||
| Sample Preparation | Individual standards (100 ppm) were combined into a final 25 ppm solution in ethyl acetate. | ||||||||
Figure 2: Highly inert RMX-5Sil MS columns improve peak shape for challenging acidic and basic drugs, allowing for more confidence in identification.
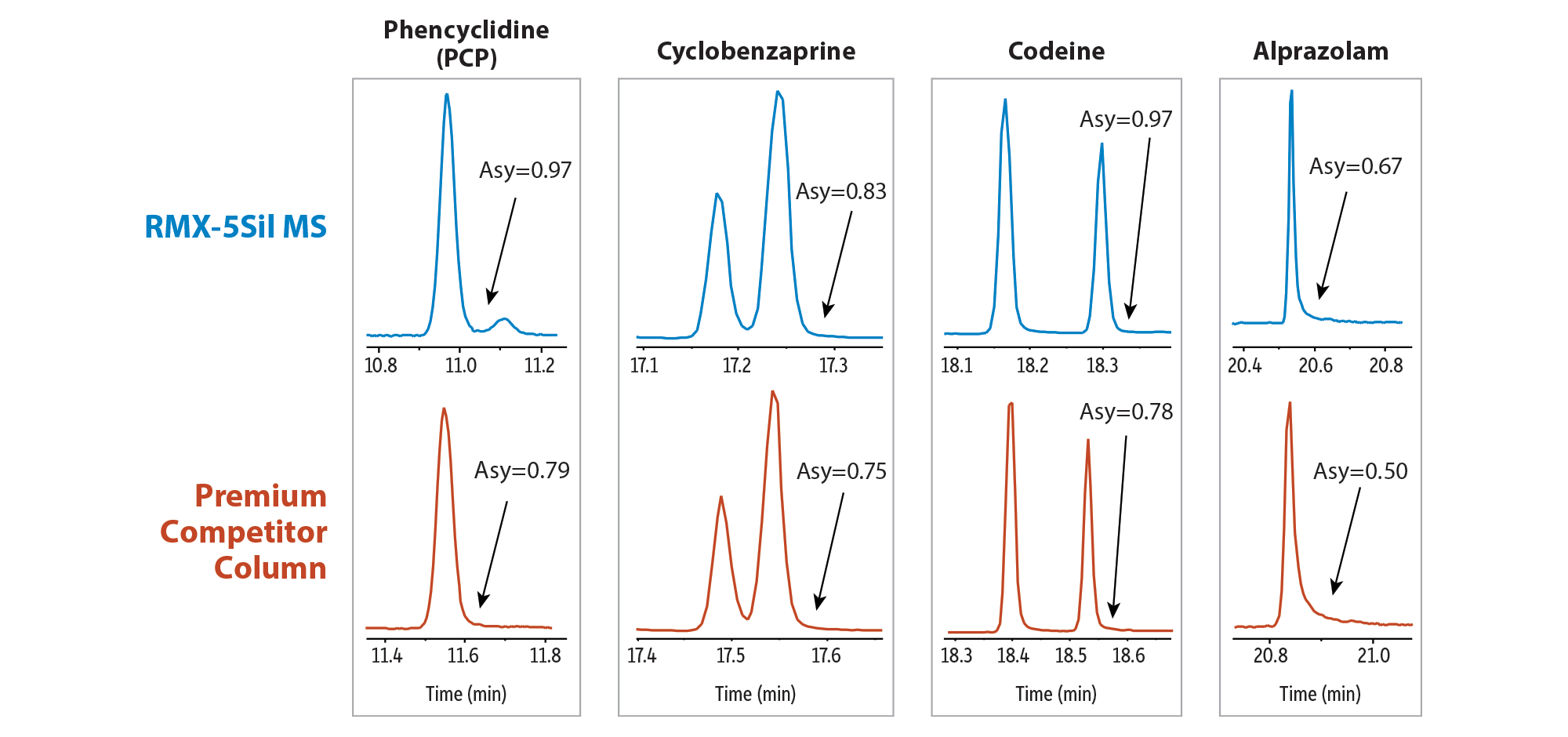
GC_CF1186
Peaks
| Peaks | Conc. (µg/mL) | tR (min) for RMX-5Sil MS | tR (min) for Premium Competitor Column | |
|---|---|---|---|---|
| 1. | Phencyclidine (PCP) | 25 | 10.97 | 11.54 |
| 2. | Cyclobenzaprine | 25 | 17.24 | 17.54 |
| 3. | Codeine | 25 | 18.29 | 18.53 |
| 4. | Alprazolam | 25 | 20.53 | 20.83 |
Conditions
| Column | See notes for names, 30 m, 0.25 mm ID, 0.25 µm |
|---|---|
| Standard/Sample | |
| Diluent: | Ethyl acetate |
| Conc.: | 25 ppm |
| Injection | |
| Inj. Vol.: | 1 µL split (split ratio 10:1) |
| Liner: | Topaz 4 mm single taper inlet liner with wool (cat.# 23303) |
| Inj. Temp.: | 250 °C |
| Split Vent Flow Rate: | 25 mL/min |
| Oven | |
| Oven Temp.: | 150 °C (hold 1.0 min) to 210 °C at 4 °C/min to 320 °C at 30 °C/min (hold 2.0 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 2.0 mL/min |
| Linear Velocity: | 54 cm/sec @ 150 °C |
| Detector | MS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mode: | Scan | ||||||||
| Scan Program: | |||||||||
| |||||||||
| Transfer Line Temp.: | 280 °C | ||||||||
| Analyzer Type: | Quadrupole | ||||||||
| Source Temp.: | 230 °C | ||||||||
| Electron Energy: | 70 eV | ||||||||
| Tune Type: | PFTBA | ||||||||
| Ionization Mode: | EI | ||||||||
| Instrument | Agilent 7890A GC & 5975C MSD | ||||||||
| Sample Preparation | Individual standards (100 ppm) were combined into a final 25 ppm in ethyl acetate. | ||||||||
| Notes | Columns tested: RMX-5Sil MS (cat.# 17323) and a premium competitor column | ||||||||