Key Highlights
- Two fast, simple direct injection methods provide baseline resolution and accurate quantitative results for bisphenols in drinking water.
- Targeted method for BPA, BPS, and BPB complies with EU directive sensitivity requirement (LLOQ of 2.5 ug/L).
- Comprehensive method for 15 bisphenols supports labs wanting to expand analyte lists in anticipation of regulatory changes.
Abstract
Bisphenols are synthetic chemicals commonly used in the production of polycarbonate plastics and epoxy resins, making them widespread environmental contaminants. Their structural similarity to estrogen allows them to act as endocrine disruptors, raising concerns about their presence in drinking water. While BPA-free products often use substitutes like BPS and BPB, these alternatives can also potentially pose health risks. This study presents a rapid, reliable method for detecting BPA, BPS, and BPB in drinking water, complying with EU regulations. A secondary method expands detection to 15 bisphenol compounds, offering a practical approach for laboratories conducting comprehensive drinking water contamination testing.
Introduction
Bisphenols are a type of man-made chemical that can be found in food and the environment as contaminants. These synthetic phenols are primarily used in the production of polycarbonate plastics and epoxy resins. Due to the ubiquitous manufacture and use of these compounds worldwide, bisphenol contaminants are likely to be found in the environment and in drinking water. In 2011, a National Institutes of Health (NIH) study published their findings that 91% of the 427 people in their study tested positive for bisphenol A in their urine samples [1]. Bisphenols share a general structure consisting of two phenol groups connected by a bridging group. This structural similarity to estrogen allows them to mimic and interfere with natural hormone functions, classifying them as endocrine disruptors [2]. Bisphenols A, B, and S are some of the compounds that have been linked to human health effects. This is especially true for bisphenol A, and regulatory authorities are moving to restrict or ban bisphenols in food contact materials and children’s toys.
Although regulations exist in some regions of the world, bisphenols and their derivatives are used in high volumes and across a wide variety of goods, which increases the likelihood of them being introduced into water supplies through environmental avenues. Bisphenol S has been used as a replacement for bisphenol A in “BPA-free” products, but it also carries health concerns and has been identified as a reproductive toxicant in California’s Proposition 65 [3].Bisphenol B is used as an alternative to both A and S in consumer goods, but there are also concerns over its endocrine-disrupting capabilities [4]. The European Union (EU) Drinking Water Directive has introduced bisphenol A as a parameter and by January of 2026 water suppliers must ensure that bisphenol A levels do not exceed the parametric limit of 2.5 µg/L in drinking water [5].
In this work, two direct injection LC-MS/MS methods for bisphenols in drinking water were established. A rapid and reliable method was developed for bisphenols A, B, and S because they are the most frequently analyzed bisphenols. This method satisfies the EU’s Drinking Water Directive and was evaluated for accuracy and precision using fortified water samples. In addition, a secondary, comprehensive method was developed to include a total of 15 bisphenol compounds, offering a proactive approach to anticipated regulatory changes. The methods presented here provide a quick and easy solution for drinking water analysis for bisphenols for labs interested in implementing or expanding contamination testing.
Experimental
LC-MS/MS Method for Bisphenols A, B, S
The chromatographic conditions are shown in Table I, and the transitions and internal standards are provided in Table II.
Table I: Analytical Conditions for Bisphenols A, B, and S
| Column | Raptor Biphenyl 50 x 3.0 mm, 2.7 µm (cat.# 9309A5E) | |
| Guard column | Raptor Biphenyl EXP guard column cartridge 5 x 3.0 mm, 2.7 µm (cat.# 9309A0253) | |
| Column temperature | 30 °C | |
| Injection volume | 10 µL | |
| Mobile phase A | Water | |
| Mobile phase B | Methanol | |
| Flow rate | 0.8 mL/min | |
| Detection | ESI (-) MS/MS | |
| Gradient | Time (min) | %B |
| 0.00 | 40 | |
| 0.50 | 40 | |
| 3.50 | 85 | |
| 3.51 | 40 | |
| 5.00 | 40 | |
| Valve position to waste | 0.00 | – |
| Valve position to MS | 0.80 | – |
| Valve position to waste | 3.51 | – |
Table II: Analyte MS Transitions for Bisphenols A, B, and S in Water Samples
| Analyte | Precursor | Product 1 | Product 2 | Internal Standard |
|---|---|---|---|---|
| Bisphenol S | 249.00 | 108.15 | 92.10 | 1 |
| Bisphenol A | 227.00 | 212.05 | 133.25 | 2 |
| Bisphenol B | 241.00 | 212.05 | 211.10 | 2 |
| Bisphenol A-d16 | 241.00 | 223.25 | – | 2 |
| Bisphenol S-d8 | 257.00 | 112.20 | – | 1 |
Comprehensive LC-MS/MS Method for Bisphenols
The chromatographic conditions are shown in Table III, and the transitions and internal standards are provided in Table IV.
Table III: Analytical Conditions for 15 Bisphenols
| Column | Raptor Biphenyl 50 x 3.0 mm, 2.7 µm (cat.# 9309A5E) | |
| Guard column | Raptor Biphenyl EXP guard column cartridge 5 x 3.0 mm, 2.7 µm (cat.# 9309A0253) | |
| Column temperature | 30 °C | |
| Injection volume | 10 µL | |
| Mobile phase A | Water | |
| Mobile phase B | Methanol | |
| Flow rate | 0.8 mL/min | |
| Detection | ESI (-) MS/MS | |
| Gradient | Time (min) | %B |
| 0.00 | 40 | |
| 1.00 | 40 | |
| 6.50 | 85 | |
| 7.00 | 85 | |
| 7.01 | 40 | |
| 8.50 | 40 | |
| Valve position to waste | 0.00 | – |
| Valve position to MS | 1.00 | – |
| Valve position to waste | 7.00 | – |
Table IV: Analyte MS Transitions for 15 Bisphenols in Water Samples
| Analyte | Precursor | Product 1 | Product 2 | Internal Standard |
|---|---|---|---|---|
| Bisphenol S-d8 | 257.00 | 112.20 | – | 1 |
| Bisphenol S | 249.00 | 108.15 | 92.10 | 1 |
| Bisphenol F | 199.00 | 93.10 | 104.85 | 2 |
| Bisphenol E | 213.00 | 197.10 | 119.05 | 2 |
| Bisphenol A-d16 | 241.00 | 223.25 | – | 2 |
| Bisphenol A | 227.00 | 212.05 | 133.25 | 2 |
| Bisphenol AF | 335.00 | 265.05 | 177.20 | 3 |
| Bisphenol B | 241.00 | 212.05 | 211.10 | 2 |
| Bisphenol C | 255.00 | 240.15 | 147.20 | 2 |
| Bisphenol AP | 289.00 | 274.15 | 273.15 | 2 |
| Bisphenol Z | 267.00 | 173.15 | 223.10 | 2 |
| Bisphenol G | 311.00 | 296.30 | 295.00 | 3 |
| Bisphenol FL | 349.00 | 256.15 | 215.20 | 2 |
| Bisphenol BP | 351.00 | 274.15 | 273.15 | 3 |
| Bisphenol M | 345.00 | 251.20 | 330.20 | 3 |
| Bisphenol P-13C4 | 349.00 | 333.20 | – | 3 |
| Bisphenol P | 345.00 | 330.15 | 315.15 | 3 |
| Bisphenol PH | 379.00 | 364.10 | 209.15 | 3 |
Standard and Sample Preparation
Calibration standards for the LC-MS/MS methods for bisphenols were prepared as follows at a range of 2.5-300 µg/L. A 30 µL aliquot of 1 mg/L isotopically labeled internal standard (IS) was mixed with 50 µL of working standard and 920 µL MS-grade water in amber vials (cat.# 21142). Five different sources of drinking water were collected locally. For accuracy and precision experiments, 1 mL water samples were fortified at 2.5 (LLOQ); 7.5 (LQC); and 75 (MQC) µg/L. All samples were capped, vortexed, and 10 µL injected into the LC-MS/MS for analysis.
Results and Discussion
Chromatographic Performance
The Raptor Biphenyl column, used under the chromatographic conditions established here, achieved full resolution for both the targeted BPA, BPB, and BPS method as well as the comprehensive 15-bisphenols method. Complete chromatographic separation is useful with MS detection because it mitigates ion suppression caused by coeluting analytes. It also allows these methods to be amenable to analysis with less expensive and non-specific fluorescence detectors, which need baseline resolution to integrate each analyte accurately. Both LC-MS/MS methods for bisphenols analysis used direct injection and had fast run times, making them ideal for high-throughput environments. The three-analyte bisphenols method has a total cycle time of just 5 minutes, and the comprehensive 15-analyte method has a cycle time of 8.5 minutes. In order to reduce both matrix effects and the amount of matrix reaching the MS, a diverter valve was used before and after compound elution to divert the eluent to waste. This step was implemented because it reduces downtime by allowing instruments to analyze more samples before maintenance is required.
Figure 1: Bisphenols A, B, and S Analyzed by LC-MS/MS
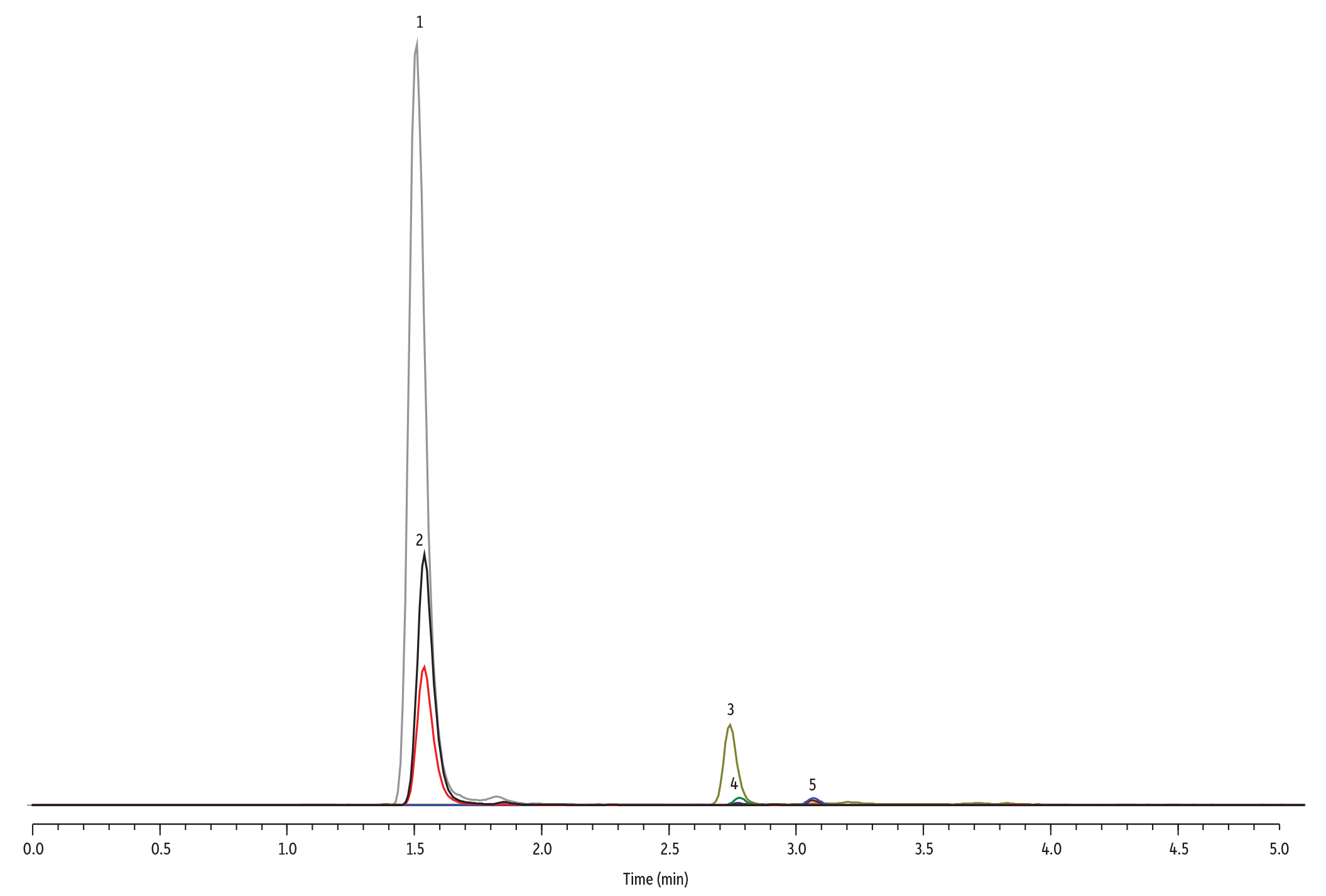
LC_EV0603
Peaks
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion 1 | Product Ion 2 | |
|---|---|---|---|---|---|---|
| 1. | Bisphenol S (BPS) | 1.57 | 2.5 | 249.0 | 108.2 | 92.1 |
| 2. | Bisphenol S-d8 | 1.57 | 30 | 257.0 | 112.2 | – |
| 3. | Bisphenol A (BPA) | 2.74 | 2.5 | 227.0 | 212.1 | 133.3 |
| 4. | Bisphenol A-d16 | 2.77 | 30 | 241.0 | 223.3 | – |
| 5. | Bisphenol B (BPB) | 3.06 | 2.5 | 241.0 | 212.1 | 211.1 |
Conditions
| Column | Raptor Biphenyl (cat.# 9309A5E) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 50 mm x 3.0 mm ID | ||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||
| Guard Column: | Raptor Biphenyl EXP guard column cartridge 5 mm, 3.0 mm ID, 2.7 µm (cat.# 9309A0253) | ||||||||||||||||||||||||
| Temp.: | 30 °C | ||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||
| Diluent: | Water | ||||||||||||||||||||||||
| Conc.: | 2.5 ng/mL | ||||||||||||||||||||||||
| Inj. Vol.: | 10 µL | ||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||
| A: | Water | ||||||||||||||||||||||||
| B: | Methanol | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Max Pressure: | 330 bar |
| Detector | Shimadzu 8060 MS/MS |
|---|---|
| Ion Source: | Electrospray |
| Ion Mode: | ESI- |
| Mode: | MRM |
| Instrument | Shimadzu Nexera X2 |
| Sample Preparation | Water samples were aliquoted into an amber short-cap vial (cat.# 21142) and fortified with analytes at 2.5 ng/mL. The samples were then capped with a 9 mm short cap (cat.# 24497) and vortexed ~30 seconds before being injection onto the LC-MS/MS for analysis. |
| Notes | The flow was directed to waste for the first 0.8 minutes of analysis and after 3.50 minutes to maintain cleanliness of the source. |
Figure 2: Comprehensive Method for 15 Bisphenols by LC-MS/MS
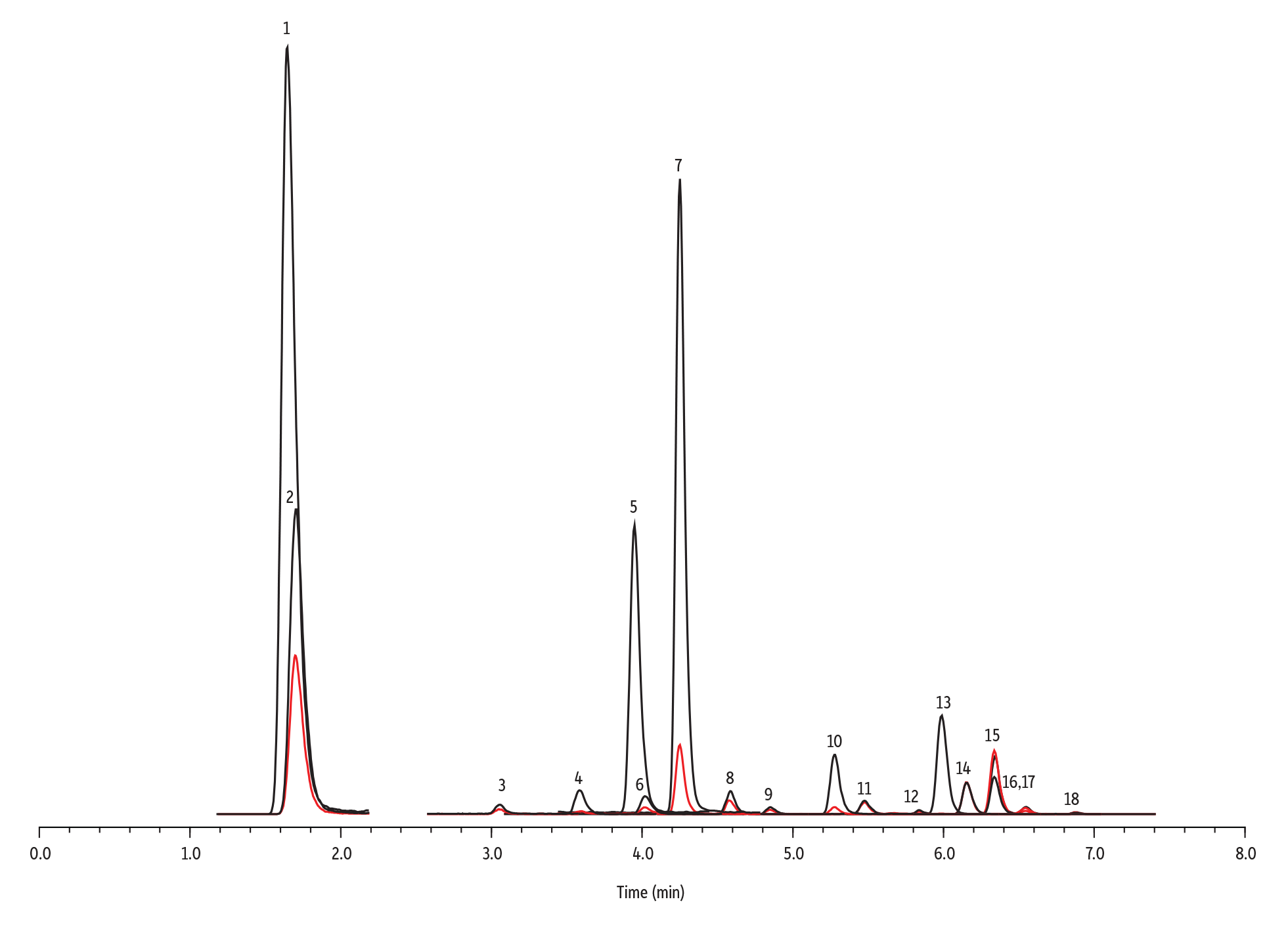
LC_EV0604
Peaks
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion 1 | Product Ion 2 | Internal Standard | |
|---|---|---|---|---|---|---|---|
| 1. | Bisphenol S-d8 | 1.65 | 30 | 257.0 | 112.2 | – | 1 |
| 2. | Bisphenol S (BPS) | 1.70 | 2.5 | 249.0 | 108.1 | 92.1 | 1 |
| 3. | Bisphenol F (BPF) | 3.06 | 2.5 | 199.0 | 93.1 | 104.9 | 2 |
| 4. | Bisphenol E (BPE) | 3.59 | 2.5 | 213.0 | 197.1 | 119.1 | 2 |
| 5. | Bisphenol A-d16 | 3.95 | 30 | 241.0 | 223.3 | – | 2 |
| 6. | Bisphenol A (BPA) | 4.02 | 2.5 | 227.0 | 212.1 | 133.3 | 2 |
| 7. | Bisphenol AF (BPAF) | 4.25 | 2.5 | 335.0 | 265.1 | 177.2 | 3 |
| 8. | Bisphenol B (BPB) | 4.59 | 2.5 | 241.0 | 212.1 | 211.1 | 2 |
| 9. | Bisphenol C (BPC) | 4.85 | 2.5 | 255.0 | 240.2 | 147.2 | 2 |
| 10. | Bisphenol AP (BPAP) | 5.28 | 2.5 | 289.0 | 274.2 | 273.2 | 2 |
| 11. | Bisphenol Z (BPZ) | 5.48 | 2.5 | 267.0 | 173.2 | 223.1 | 2 |
| 12. | Bisphenol G (BPG) | 5.84 | 2.5 | 311.0 | 296.3 | 295.0 | 3 |
| 13. | Bisphenol FL (BPFL) | 5.99 | 2.5 | 349.0 | 256.2 | 215.2 | 2 |
| 14. | Bisphenol BP (BPBP) | 6.15 | 2.5 | 351.0 | 274.2 | 273.2 | 3 |
| 15. | Bisphenol M (BPM) | 6.34 | 2.5 | 345.0 | 251.2 | 330.2 | 3 |
| 16. | Bisphenol P-13C4 | 6.50 | 30 | 349.0 | 333.2 | – | 3 |
| 17. | Bisphenol P (BPP) | 6.55 | 2.5 | 345.0 | 330.2 | 315.2 | 3 |
| 18. | Bisphenol PH (BPPH) | 6.87 | 2.5 | 379.0 | 364.1 | 209.2 | 3 |
Conditions
| Column | Raptor Biphenyl (cat.# 9309A5E) | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 50 mm x 3.0 mm ID | ||||||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||||||
| Guard Column: | Raptor Biphenyl EXP guard column cartridge 5 mm, 3.0 mm ID, 2.7 µm (cat.# 9309A0253) | ||||||||||||||||||||||||||||
| Temp.: | 30 °C | ||||||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||||||
| Diluent: | Water | ||||||||||||||||||||||||||||
| Conc.: | 2.5 ng/mL | ||||||||||||||||||||||||||||
| Inj. Vol.: | 10 µL | ||||||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||||||
| A: | Water | ||||||||||||||||||||||||||||
| B: | Methanol | ||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
| Max Pressure: | 329 bar |
| Detector | Shimadzu 8060 MS/MS |
|---|---|
| Ion Source: | Electrospray |
| Ion Mode: | ESI- |
| Mode: | MRM |
| Instrument | Shimadzu Nexera X2 |
| Sample Preparation | Water samples were aliquoted into an amber short-cap vial (cat.# 21142) and fortified with analytes at 2.5 ng/mL. The samples were then capped with a 9 mm short cap (cat.# 24497) and vortexed ~30 seconds before being injection onto the LC-MS/MS for analysis. |
| Notes | The flow was directed to waste for the first minute of analysis and after 7.00 minutes to maintain cleanliness of the source. |
Internal Standard Assessment
Two isotopically labeled internal standards were examined for the analysis of bisphenols A, B, and S to determine if they would effectively compensate for matrix effects. The results showed excellent accuracy and precision. However, when the two IS were used for the comprehensive 15 bisphenols method, the results showed that using only two internal standards returned poor quantitative results for the mid-to-late eluting compounds. The optimization of valve switching helped with accuracy and precision for bisphenols F and E, but it did not improve quantitative results for bisphenols AF, G, BP, M, P, and PH. A third internal standard, bisphenol P-13C4, was introduced into the method and acceptable precision and accuracy values were obtained for all 15 bisphenols.
Linearity
Both LC-MS/MS methods for bisphenols analysis produced linear calibrations. Using a 1/x weighted linear regression, bisphenols A, B, and S showed acceptable linearities with r2 values of >0.993 and deviations of <20% across a calibration range of 2.5-300 µg/L. The comprehensive method also used a linear regression of 1/x with acceptable linearities of r2 >0.991 and deviations of <20% across calibration range of 2.5-300 µg/L for all compounds except bisphenol PH (5-150 µg/L); bisphenol G (5-100 µg/L); and bisphenol AF (2.5-150 µg/L).
Accuracy and Precision
Drinking water was collected locally and fortified with bisphenols A, B, and S at 2.5 (LLOQ); 7.5 (LQC); and 75 (MQC) µg/L. Three batches were analyzed over three days for a total of nine replicates. The results can be seen in Table V. Accuracy ranged from 82.4-113.7% for bisphenols A, B, S for intraday results and 86.6-108.3% for interday percent recovery. Precision measured by %RSD values was ≤11.2% for intraday results and ≤6.6% for interday results.
Table V: Accuracy and Precision Results for Bisphenols A, B, and S Method
| Day 1 | Day 2 | Day 3 | Interday | |||||||||
| Water Sample | Target Conc. (µg/L) | Calc. Conc. (µg/L) | % Recovery | %RSD | Calc. Conc. (µg/L) | % Recovery | %RSD | Calc. Conc. (µg/L) | % Recovery | %RSD | Average | %RSD |
| Bisphenol S | ||||||||||||
| 1 Drinking Water | 2.5 | 2.2 | 86.8 | 4.0 | 2.3 | 90.6 | 2.2 | 2.4 | 95.7 | 1.2 | 91.0 | 4.9 |
| 7.5 | 7.9 | 106.0 | 2.5 | 7.8 | 103.8 | 3.6 | 7.9 | 105.2 | 1.5 | 105.0 | 1.1 | |
| 75.0 | 78.0 | 103.9 | 0.9 | 75.5 | 100.7 | 4.1 | 76.8 | 102.4 | 1.5 | 102.3 | 1.6 | |
| 2 Ice Machine Water | 2.5 | 2.1 | 84.8 | 2.9 | 2.3 | 91.2 | 4.3 | 2.4 | 95.0 | 2.9 | 90.3 | 5.7 |
| 7.5 | 7.6 | 101.3 | 5.8 | 7.9 | 104.7 | 2.9 | 7.8 | 104.2 | 1.8 | 103.4 | 1.8 | |
| 75.0 | 77.6 | 103.5 | 5.7 | 78.7 | 104.9 | 3.3 | 75.7 | 100.9 | 1.9 | 103.1 | 2.0 | |
| 3 Drinking water | 2.5 | 2.1 | 84.0 | 3.0 | 2.3 | 91.1 | 5.4 | 2.4 | 95.8 | 1.6 | 90.3 | 6.6 |
| 7.5 | 7.7 | 102.4 | 7.4 | 7.9 | 105.5 | 4.7 | 7.9 | 105.1 | 1.2 | 104.3 | 1.6 | |
| 75.0 | 80.5 | 107.3 | 2.8 | 80.7 | 107.6 | 5.2 | 77.0 | 102.6 | 1.3 | 105.9 | 2.6 | |
| 4 Drinking water | 2.5 | 2.1 | 84.6 | 7.6 | 2.2 | 86.3 | 4.5 | 2.2 | 88.9 | 2.6 | 86.6 | 2.5 |
| 7.5 | 7.6 | 101.1 | 2.6 | 7.3 | 97.1 | 4.4 | 7.4 | 98.0 | 6.4 | 98.7 | 2.1 | |
| 75.0 | 78.0 | 103.9 | 2.2 | 78.1 | 104.1 | 1.9 | 78.0 | 104.0 | 2.0 | 104.0 | 0.1 | |
| 5 Drinking water | 2.5 | 2.1 | 82.4 | 4.4 | 2.3 | 92.6 | 5.3 | 2.3 | 91.5 | 2.4 | 88.8 | 6.3 |
| 7.5 | 7.6 | 101.4 | 4.8 | 8.1 | 108.2 | 2.8 | 7.6 | 101.5 | 2.3 | 103.7 | 3.8 | |
| 75.0 | 77.8 | 103.7 | 2.0 | 85.3 | 113.7 | 3.9 | 76.1 | 101.5 | 1.9 | 106.3 | 6.1 | |
| Bisphenol B | ||||||||||||
| Day 1 | Day 2 | Day 3 | Interday | |||||||||
| Water Sample | Target Conc. (µg/L) | Calc. Conc. (µg/L) | % Recovery | %RSD | Calc. Conc. (µg/L) | % Recovery | %RSD | Calc. Conc. (µg/L) | % Recovery | %RSD | Average | %RSD |
| 1 Drinking Water | 2.5 | 2.7 | 106.3 | 2.6 | 2.7 | 106.6 | 6.5 | 2.6 | 102.4 | 4.7 | 105.1 | 2.2 |
| 7.5 | 7.3 | 97.0 | 2.1 | 7.3 | 97.8 | 2.9 | 7.0 | 92.8 | 4.1 | 95.9 | 2.8 | |
| 75.0 | 72.0 | 96.0 | 6.6 | 69.6 | 92.8 | 3.4 | 74.4 | 99.2 | 11.2 | 96.0 | 3.3 | |
| 2 Ice Machine Water | 2.5 | 2.5 | 100.1 | 5.4 | 2.4 | 96.3 | 4.7 | 2.5 | 101.4 | 1.7 | 99.3 | 2.7 |
| 7.5 | 7.2 | 95.5 | 2.3 | 7.0 | 93.2 | 5.3 | 7.1 | 94.4 | 10.6 | 94.4 | 1.3 | |
| 75.0 | 69.5 | 92.7 | 2.2 | 65.7 | 87.6 | 1.8 | 70.2 | 93.7 | 5.3 | 91.3 | 3.5 | |
| 3 Drinking water | 2.5 | 2.5 | 101.9 | 3.8 | 2.7 | 108.0 | 0.1 | 2.7 | 106.8 | 3.7 | 105.6 | 3.0 |
| 7.5 | 7.4 | 98.1 | 1.7 | 7.3 | 97.2 | 4.3 | 7.2 | 96.2 | 3.2 | 97.2 | 1.0 | |
| 75.0 | 68.2 | 91.0 | 3.1 | 71.1 | 94.8 | 2.4 | 72.6 | 96.8 | 4.0 | 94.2 | 3.2 | |
| 4 Drinking water | 2.5 | 2.7 | 106.4 | 5.8 | 2.7 | 110.0 | 1.7 | 2.7 | 108.6 | 7.2 | 108.3 | 1.7 |
| 7.5 | 7.8 | 104.2 | 3.1 | 7.7 | 103.0 | 2.3 | 7.5 | 100.4 | 5.6 | 102.5 | 1.9 | |
| 75.0 | 79.6 | 106.1 | 3.0 | 72.7 | 96.9 | 1.7 | 71.6 | 95.5 | 3.4 | 99.5 | 5.8 | |
| 5 Drinking water | 2.5 | 2.7 | 107.7 | 1.7 | 2.6 | 102.4 | 7.2 | 2.5 | 101.7 | 0.2 | 103.9 | 3.1 |
| 7.5 | 7.5 | 100.5 | 2.6 | 7.4 | 99.0 | 2.5 | 6.7 | 89.9 | 6.0 | 96.5 | 6.0 | |
| 75.0 | 71.4 | 95.2 | 2.4 | 70.6 | 94.1 | 1.9 | 66.0 | 88.0 | 1.7 | 92.4 | 4.2 | |
| Bisphenol A | ||||||||||||
| Day 1 | Day 2 | Day 3 | Interday | |||||||||
| Water Sample | Target Conc. (µg/L) | Calc. Conc. (µg/L) | % Recovery | %RSD | Calc. Conc. (µg/L) | % Recovery | %RSD | Calc. Conc. (µg/L) | % Recovery | %RSD | Average | %RSD |
| 1 Drinking Water | 2.5 | 2.8 | 110.1 | 7.5 | 2.5 | 101.0 | 8.4 | 2.6 | 105.1 | 2.9 | 105.4 | 4.3 |
| 7.5 | 7.5 | 100.2 | 6.4 | 7.6 | 102.0 | 0.9 | 7.2 | 96.0 | 3.9 | 99.4 | 3.1 | |
| 75.0 | 72.9 | 97.1 | 3.6 | 71.4 | 95.2 | 3.0 | 76.9 | 102.6 | 5.8 | 98.3 | 3.9 | |
| 2 Ice Machine Water | 2.5 | 2.5 | 99.2 | 4.3 | 2.7 | 107.4 | 8.1 | 2.6 | 103.9 | 9.4 | 103.5 | 4.0 |
| 7.5 | 7.6 | 101.6 | 2.9 | 7.7 | 102.4 | 5.7 | 7.7 | 102.3 | 6.1 | 102.1 | 0.4 | |
| 75.0 | 71.3 | 95.1 | 4.6 | 73.6 | 98.1 | 3.3 | 73.8 | 98.4 | 1.4 | 97.2 | 1.9 | |
| 3 Drinking water | 2.5 | 2.7 | 108.7 | 7.7 | 2.6 | 104.1 | 1.5 | 2.5 | 100.6 | 4.8 | 104.5 | 3.9 |
| 7.5 | 7.8 | 104.0 | 3.6 | 7.4 | 98.2 | 3.6 | 7.4 | 98.7 | 3.1 | 100.3 | 3.2 | |
| 75.0 | 72.6 | 96.8 | 2.9 | 75.5 | 100.6 | 4.1 | 76.5 | 102.0 | 3.3 | 99.8 | 2.7 | |
| 4 Drinking water | 2.5 | 2.6 | 102.7 | 5.4 | 2.5 | 101.3 | 3.2 | 2.6 | 106.0 | 8.9 | 103.3 | 2.3 |
| 7.5 | 8.0 | 107.0 | 3.4 | 7.6 | 100.7 | 3.5 | 7.6 | 101.8 | 5.3 | 103.2 | 3.3 | |
| 75.0 | 75.3 | 100.4 | 3.1 | 75.0 | 100.0 | 2.8 | 73.4 | 97.8 | 5.4 | 99.4 | 1.4 | |
| 5 Drinking water | 2.5 | 2.5 | 100.5 | 6.2 | 2.7 | 107.3 | 3.1 | 2.5 | 99.4 | 3.2 | 102.4 | 4.2 |
| 7.5 | 7.7 | 102.2 | 6.1 | 7.4 | 98.4 | 2.5 | 7.1 | 94.4 | 7.7 | 98.4 | 4.0 | |
| 75.0 | 73.3 | 97.7 | 2.1 | 73.7 | 98.3 | 0.2 | 71.3 | 95.1 | 3.9 | 97.0 | 1.8 | |
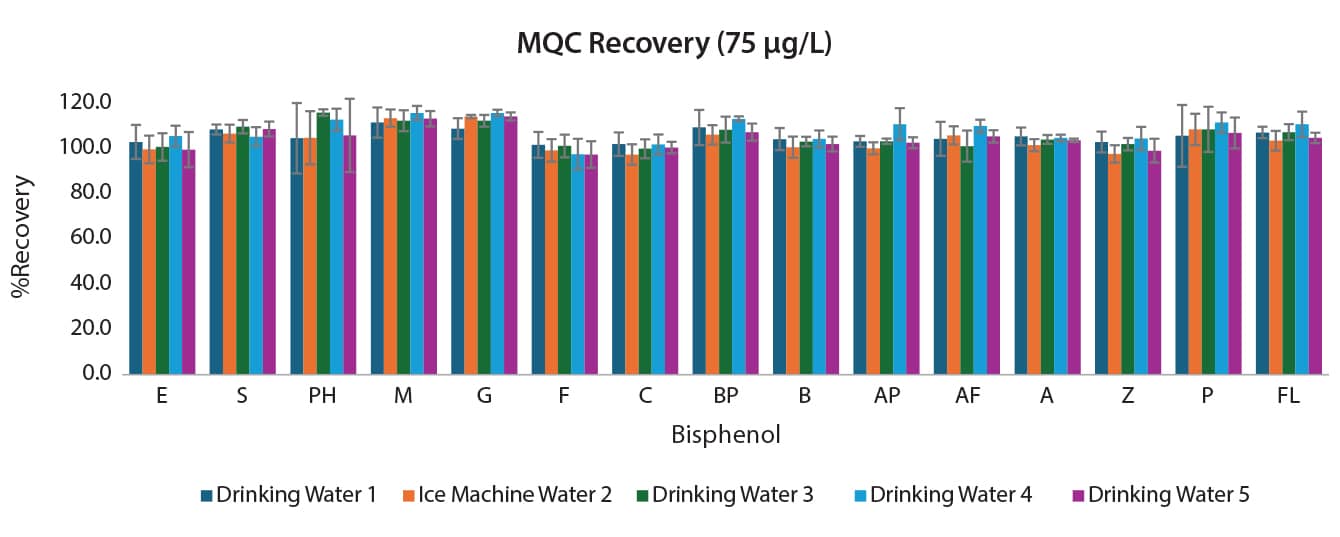
The comprehensive method also had validation and accuracy experiments performed. Water collected locally was fortified with 15 bisphenols at 2.5; 7.5; and 75 µg/L. Three batches were analyzed over three days for a total of nine replicates. The results can be seen in Figures 3, 4, and 5. Accuracy ranged from 88.9-115.9% for the comprehensive method for interday percent recovery. Precision as measured by %RSD was ≤17.3% for interday results (n=9).
Figure 3: Interday Accuracy and Precision for the Comprehensive Bisphenols Method at the LLOQ (2.5 µg/L) (n=9) (Note that recovery at 2.5 µg/L was not assessed for bisphenols PH and G due to their higher LLOQs.)
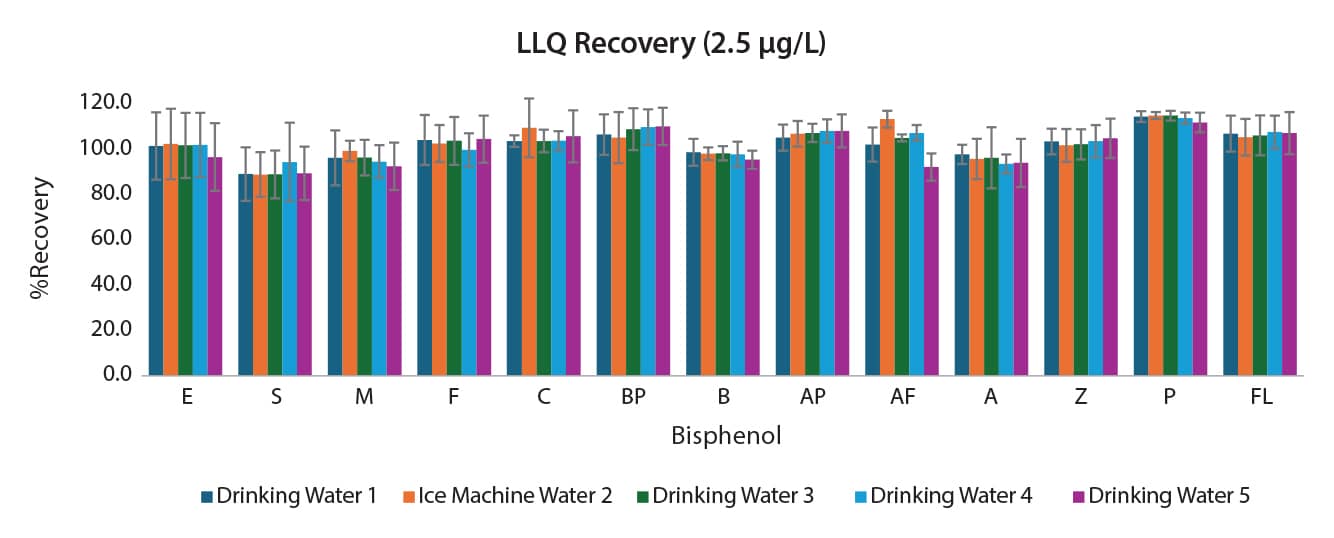
Figure 4: Interday Accuracy and Precision for the Comprehensive Bisphenols Method at the LQC (7.5 µg/L) (n=9)
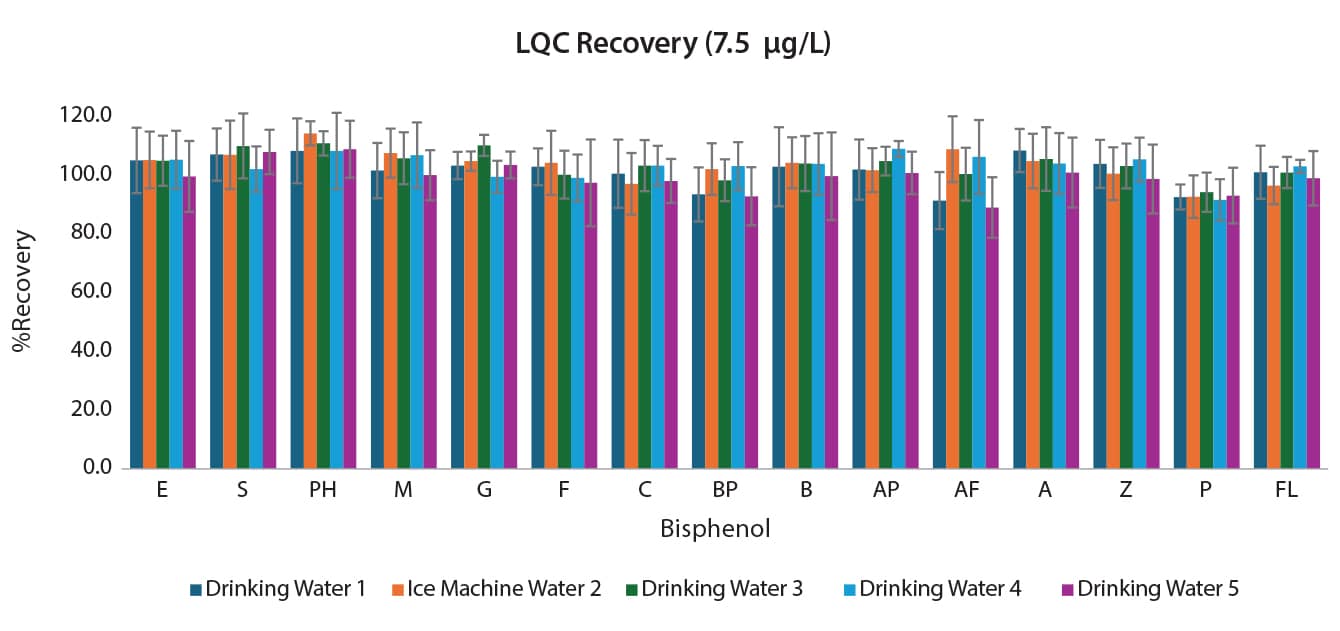
Figure 5: Interday Accuracy and Precision for the Comprehensive Bisphenols Method for at the MQC (75 µg/L) (n=9)
Conclusion
In this study, two robust LC-MS/MS methods for bisphenols analysis in drinking water were developed and both generated good quantitative results. A direct injection approach was utilized to streamline sample preparation, and the two complementary analytical methods produced complete chromatographic separation of all compounds in fast analysis times that are conducive to high-throughput testing. One method was optimized for targeted analysis of BPA, BPS, and BPB because they are the most commonly tested bisphenols. The other method was optimized for 15 bisphenols to support labs wanting to expand into more comprehensive analyte lists in anticipation of regulatory changes. The developed methods meet the sensitivity requirements outlined in the EU’s Drinking Water Directive, offering a practical solution for routine monitoring of bisphenol contamination.
References
- Ye. X; Wong, L.; Bishop, A. M.; Calafat, A. M. Variability of Urinary Concentrations of Bisphenol A in Spot Samples, First Morning Voids, and 24-Hour Collections. Environ Health Perspect. 2011 Mar 15;119(7):983–988. doi: 10.1289/ehp.1002701.
- M.E. Cull and L.M. Winn; Bisphenol A and its potential mechanism of action for reproductive toxicity. Toxicology 511 (2025) 154040.
- Bisphenol S (BPS) Added To Proposition 65 List Following 2023 Meeting of The Developmental and Reproductive Toxicant Identification Committee. State of California. 2014 Jan 23 Bisphenol S (BPS) Added to Proposition 65 List Following 2023 Meeting of the Developmental and Reproductive Toxicant Identification Committee – OEHHA. Accessed June 27, 2025.
- Bisphenol B, an endocrine disruptor for humans and the environment. Anses. 2021 Sept. 3 Bisphenol B, an endocrine disruptor for humans and the environment | Anses – Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail. Accessed June 27, 2015.
- EurEau position paper : Bisphenol-A and drinking water. Water News Europe. 2025 April 28 EurEau position paper: Bisphenol-A and drinking water • Water News Europe. Accessed July 28, 2025.








