
This question pops up a number of times every year. The joking answer my former colleague Dick Zwiep (when we both worked at Chrompack time) used to make was: No and No-two!
The challenge is not the N2O, but the NO and NO2. NO and NO2 are involved in several reactions/equilibria, that makes the quantification challenging.
N2O (Nitrous Oxide) , also known as “laughing gas”, is quite easy to analyze via GC. One can do that on a porous polymer type phase, like the Rt Q-BOND, see fig.1. The N2O elutes quite fast, meaning that a splitted injection is preferred. In this application a 0.25mm PLOT column was used as the detector was an MS. N2O is also often measured via ECD.
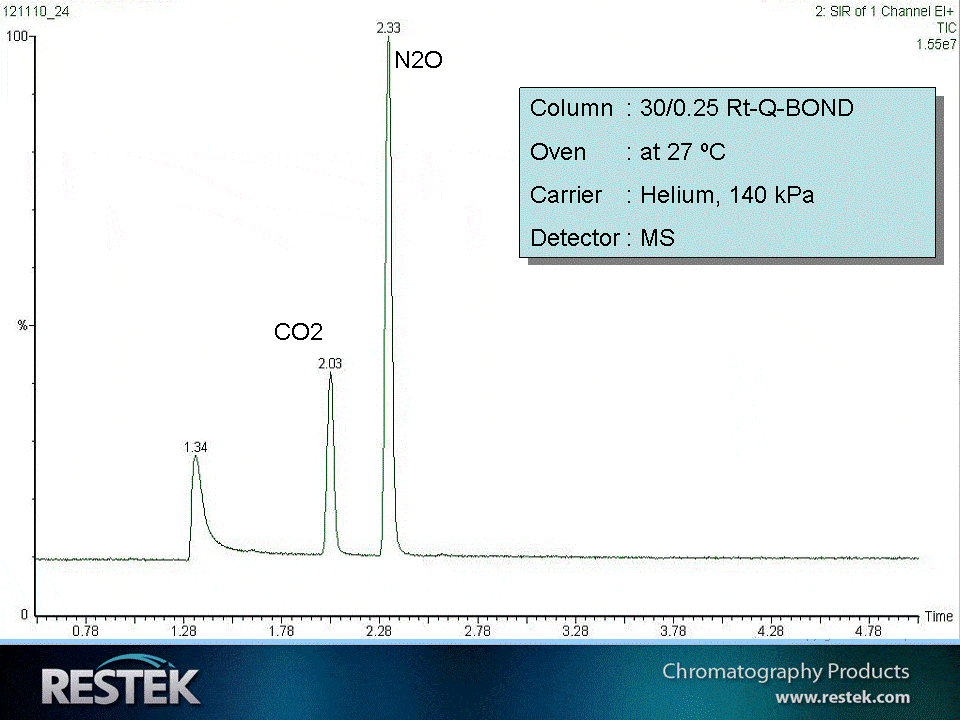
Fig.1 Nitrous oxide elutes as a symmetrical peak from a Rt-Q-BOND porous polymer.
NO, (Nitric Oxide), is more challenging to measure. When exposed to oxygen, NO is converted into NO2 (Nitrogen Dioxide), which has a dark brown color.
2 NO + O2 → 2 NO2
This is also one of the challenges that is faced. NO will immediate react with O2. If O2 is absent, NO will elute from most columns, but it will show minimal retention. Even on a porous polymer it co-elutes with permanent gases.
It is retained on Molecular sieve type adsorbents and can be measured, but peak shapes are usually not symmetrical, which challenges elution and detection of low levels.
Some more models of chemical kinetics on NO/NO2 can be found here: http://www.chem.tamu.edu/rgroup/hughbanks/courses/102H/lecturenotes/class12-1.pdf
NO2, (Nitric dioxide), is most challenging to analyze via GC. NO2 exists in equilibrium with dinitrogen tetroxide (N2O4):
2 NO2 <=> N2O4
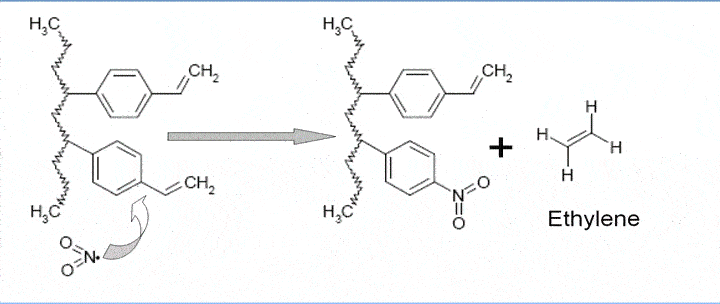
Fig.2 Reactivity of porous polymers based on vinylbenzene, with NO2 makes quantitation difficult
Few applications are out there. Most obvious would be to use porous polymers for this, but the Nitric dioxide reacts with the porous polymer forming an ethylene peak, see fig.2,. This maybe a way to measure NO2 indirectly by converting the NO2 into ethylene in a hot inlet, however, the conversion is believed not to be quantitative.
For low level detection one can use MS, ECD, PDD ands also Cheluminescence detection. TCD is also possible, but only at high ppm levels


