In my previous chiral column blogs, I’ve discussed how they compare, how to optimize the separations and column capacity. This blog focuses on individual columns and specific separations. We’ll start with Rt-βDEXm, Rt-βDEXsm and Rt-βDEXse (Table 1).
Table 1: Resolution of selected chiral compounds by Rt-βDEXm, Rt-βDEXsm and Rt-βDEXse
| Rt-βDEXm | Rt-βDEXsm | Rt-βDEXse | |
| α-pinene | 3.89 | 3.14 | 0.82 |
| limonene | 1.53 | 5.60 | 3.10 |
| linalool | 1.01 | 3.10 | 5.96 |
| α-terpineol | 1.84 | 4.72 | 5.20 |
| isoborneol | 2.20 | 3.76 | 3.35 |
| menthol | 1.73 | 1.24 | 1.24 |
| 2,3-butanediol | 2.94 | 6.44 | 7.10 |
| 1-phenylethanol | 6.93 | 7.52 | 6.52 |
| α-ionone | 3.45 | 5.67 | 3.31 |
| menthone | 4.11 | 0.59 | 5.76 |
| ethyl-2-methylbutyrate | 1.00 | 3.94 | 4.66 |
| linalyl acetate | ns | ns | 2.36 |
| styrene oxide | 3.34 | 4.53 | 10.77 |
| trans-linalool oxide | 9.74 | 9.71 | 2.96 |
| cis-linalool oxide | 5.65 | 6.06 | 4.28 |
All chromatograms were collected using the following method: Oven temp.: 40°C (hold 1 min.) to 230°C @ 2°C/min. (hold 3 min.); Carrier gas: hydrogen; 80cm/sec. set @ 40°C; Detector: FID set @ 220°C.
Rt-βDEXm
Let’s start with the most basic chiral column, the Rt-βDEXm, which is permethylated β-cyclodextrin added into midpolarity cyano/phenyl polymer (the same as 1701 phase). Based on our table (Table 1), Rt-βDEXm resolves well α-pinene, menthol, 1-phenylethanol, methone and linalool oxides. In addition, this column resolved menthol better than any other chiral column in our study. Figure 1 shows the separation of menthol (A) and menthone (B) on Rt-βDEXm, because these two compounds are commonly found together in mint oils.
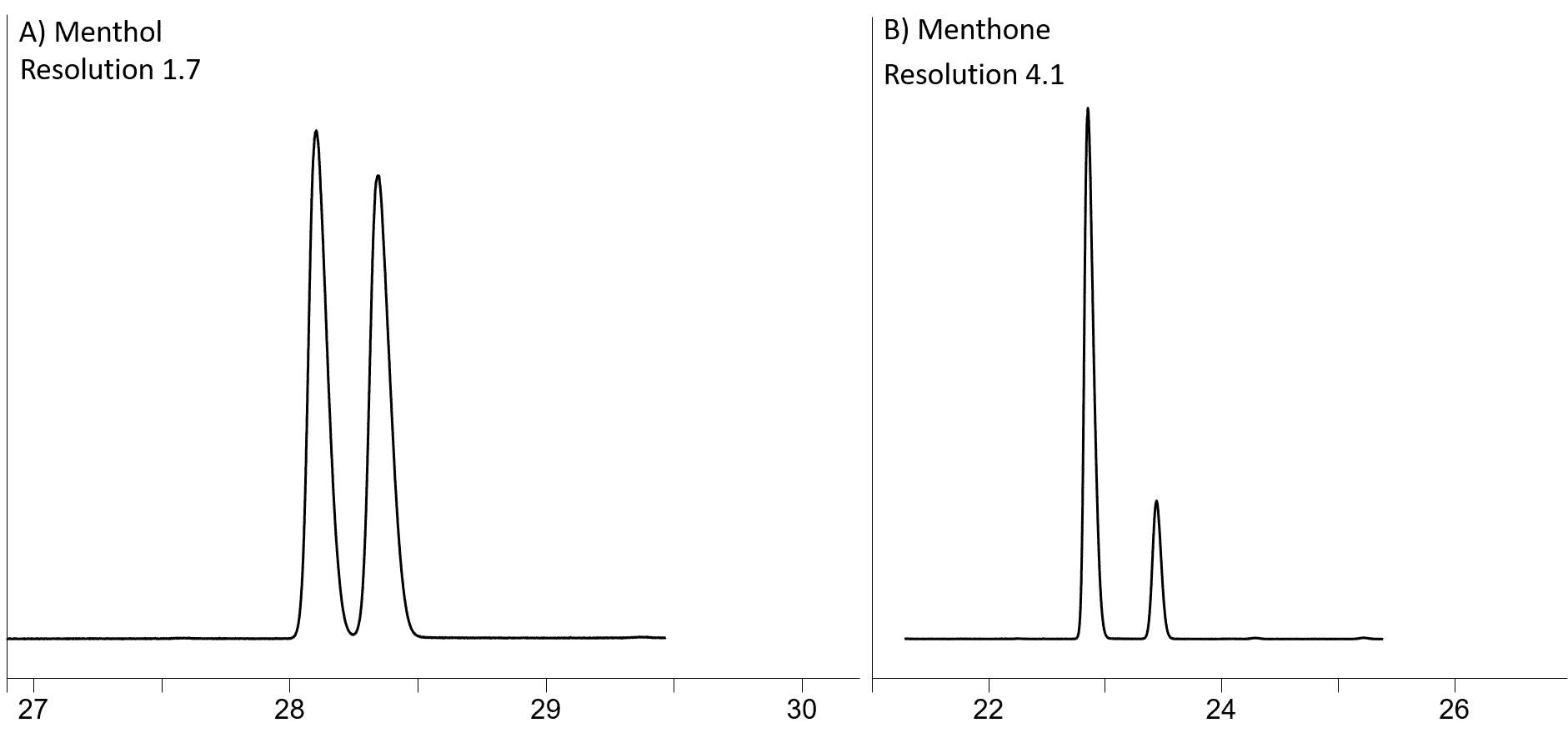
Figure 1: Methol (A) and Menthone (B) resolution on Rt-βDEXm (30m, 0.32mm ID, 0.25µm; cat. # 13101)
Rt-βDEXsm
Rt-βDEXsm separates 20 of the 21 tested compounds, with 16 being baseline resolved. This column provides very good enantiomeric separation (Table 1) of α-pinene, α-ionone (Fig 2A), α-terpineol (Fig 2B), isoborneol (Fig 2C), 1-phenylethanol, 2,3-butanediol and linalool oxides (Fig 3).
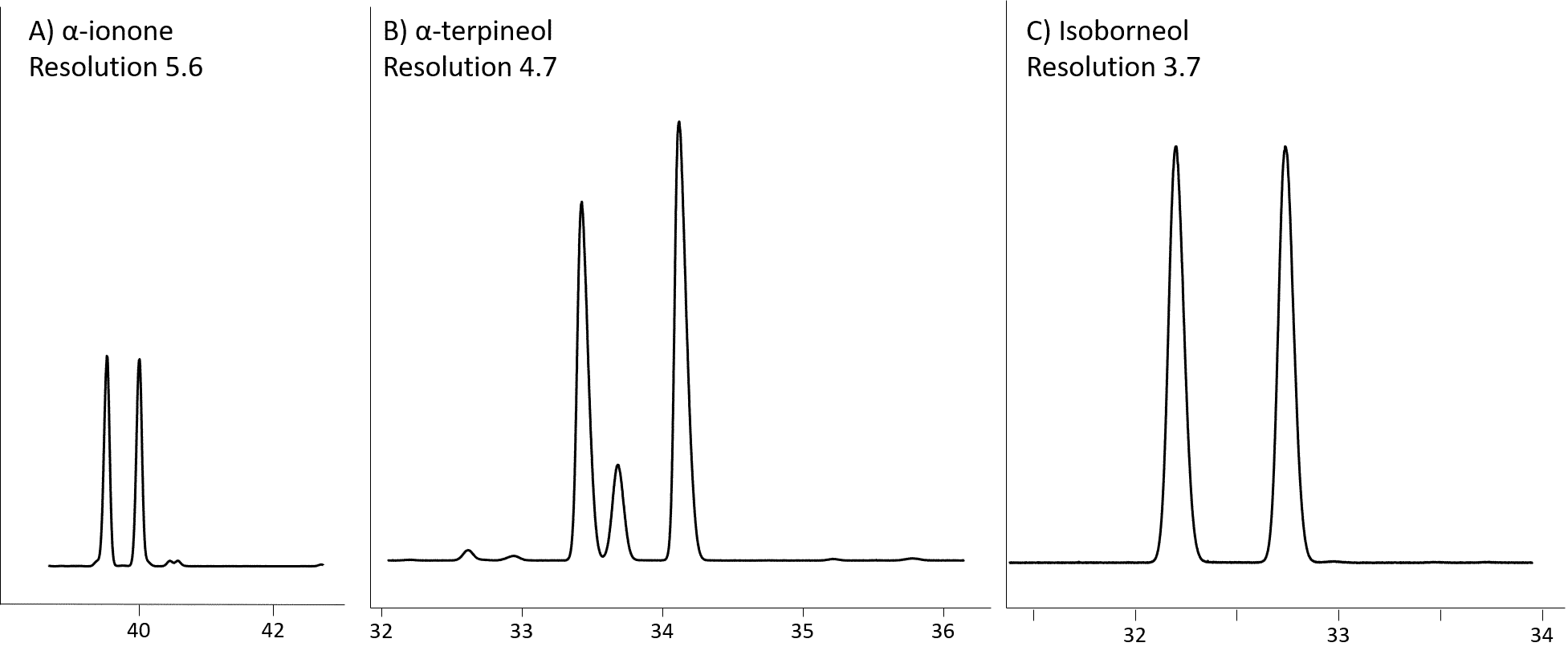
Figure 2: α-ionone (A), α-terpineol (B) and isoborneol (C) resolution on Rt-βDEXsm (30m, 0.32mm ID, 0.25µm; cat. # 13104)
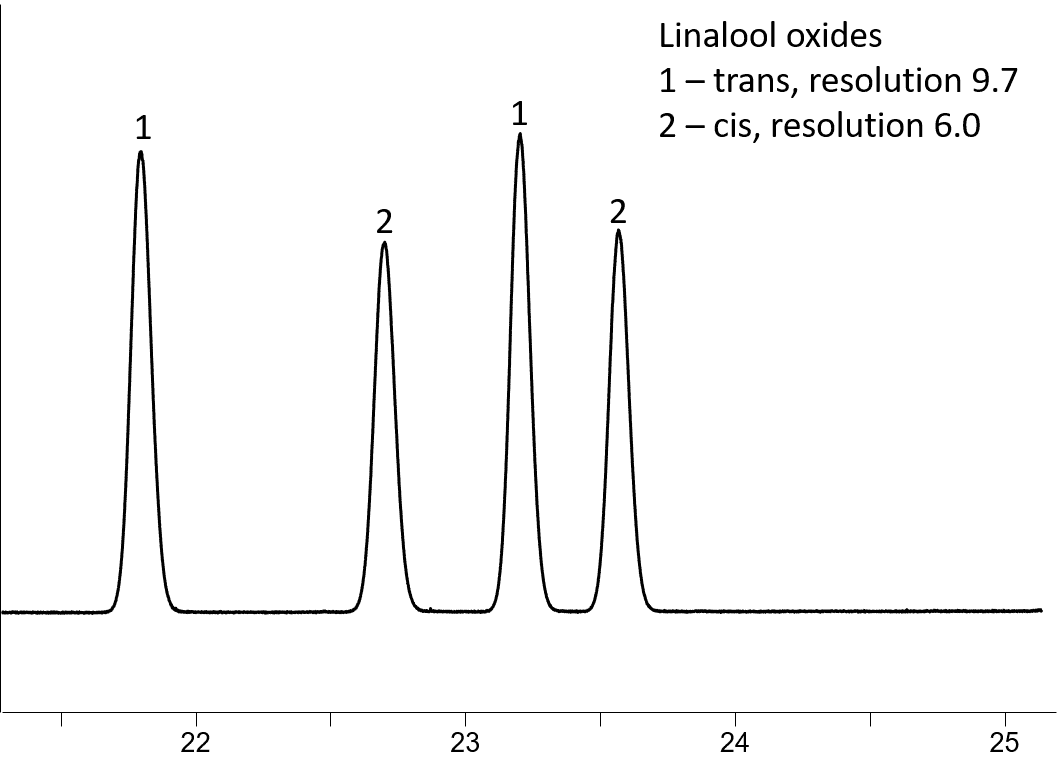
Figure 3: Trans-linalool oxide (1) and cis-linalool oxide (2) resolution on Rt-βDEXsm (30m, 0.32mm ID, 0.25µm; cat. # 13104)
Rt-βDEXse
The Rt-βDEXse is similar in performance to the Rt-βDEXsm, but it provides better resolution for limonene, α-terpineol, linalool, linalyl acetate (Fig 4), ethyl-2-methylbutyrate (Fig 5A), 2,3-butanediol, menthone (Fig 5B) and styrene oxides.
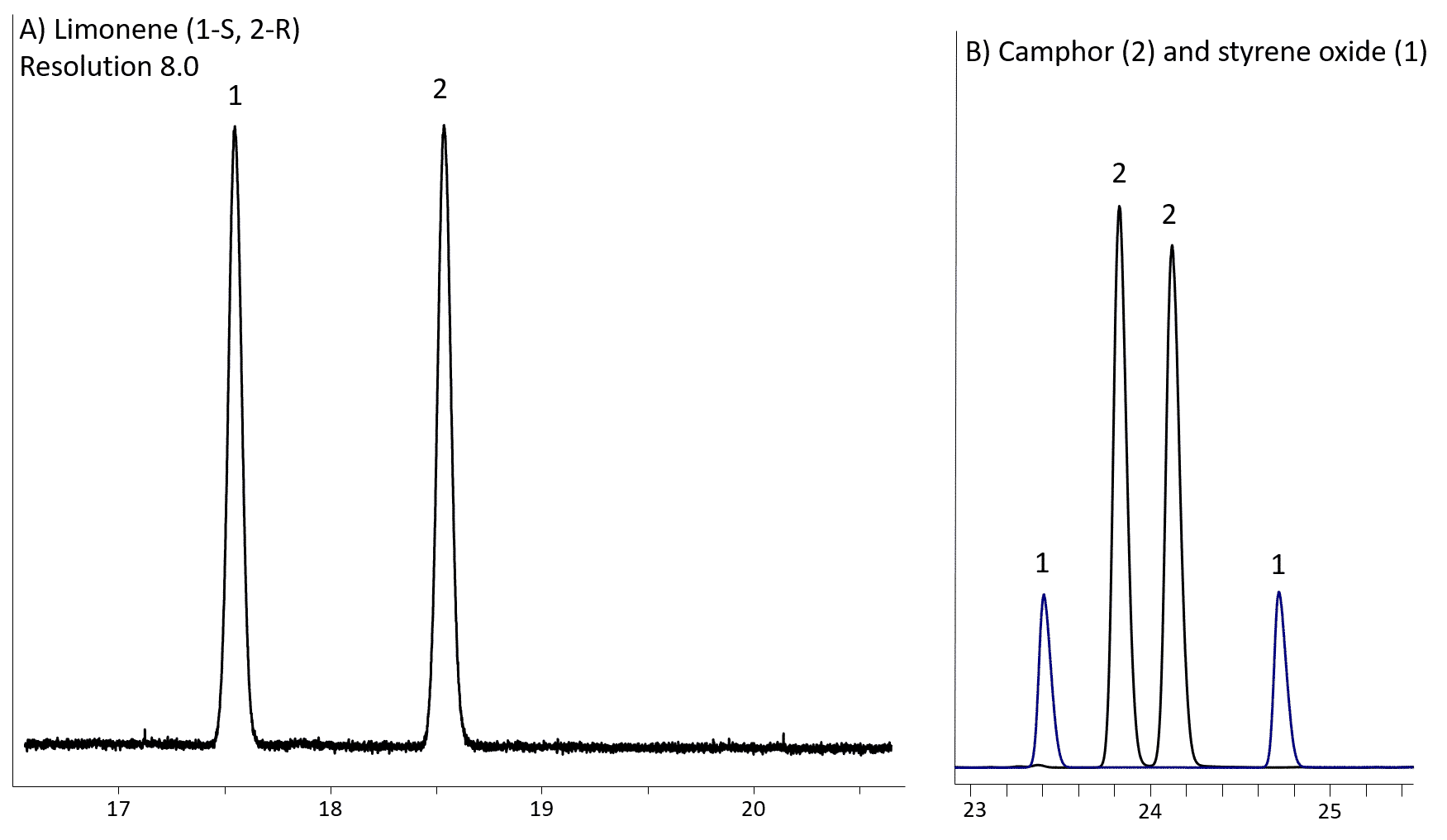
Figure 4: Linalool and linalyl acetate resolution on Rt-βDEXse (30m, 0.32mm ID, 0.25µm; cat. # 13106)
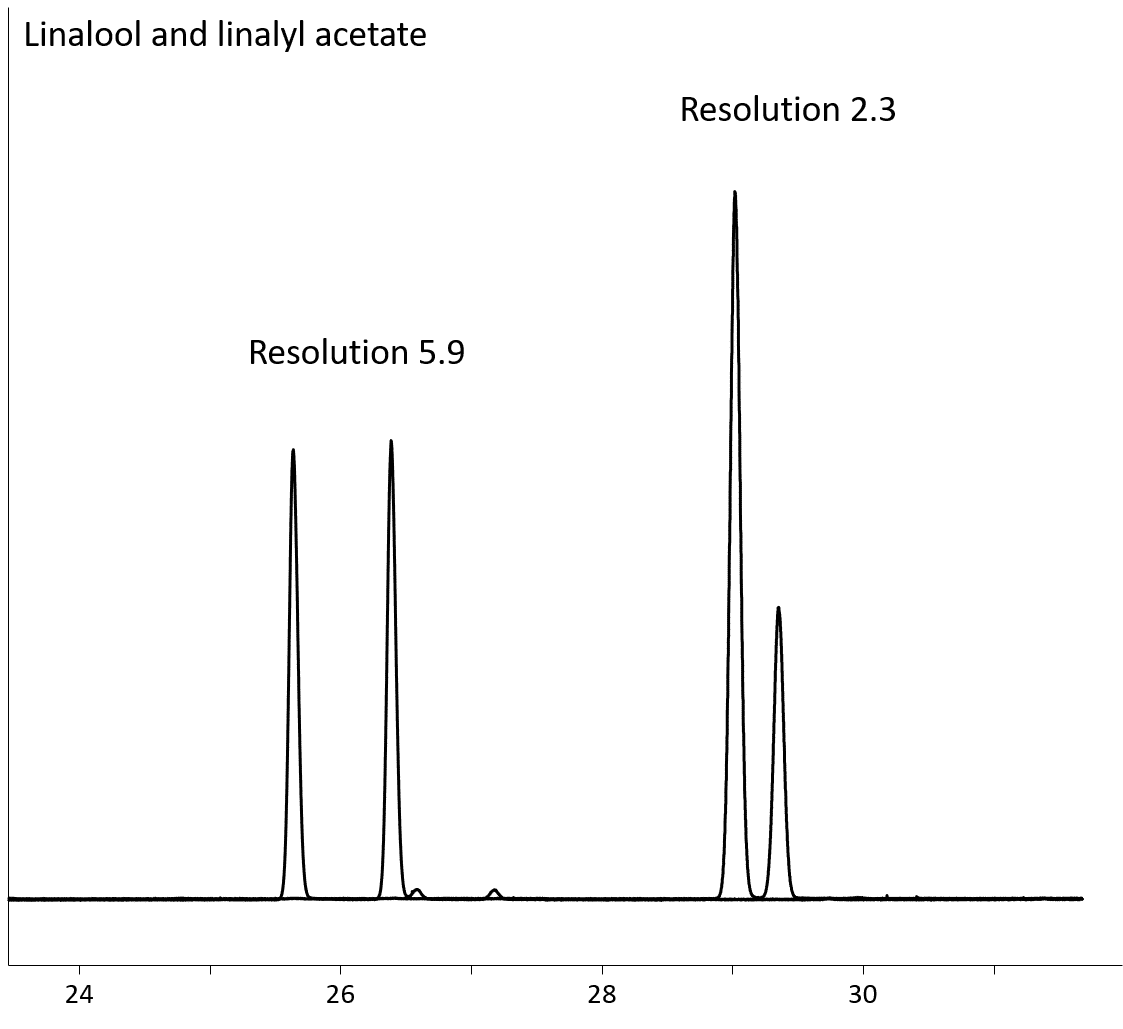
Figure 5: Ethyl2-methylbutyrate (A) and menthone (B) resolution on Rt-βDEXse (30m, 0.32mm ID, 0.25µm; cat. # 13106)
Sometimes extensive separation results in overlap of enantiomeric pairs (Fig 6B).
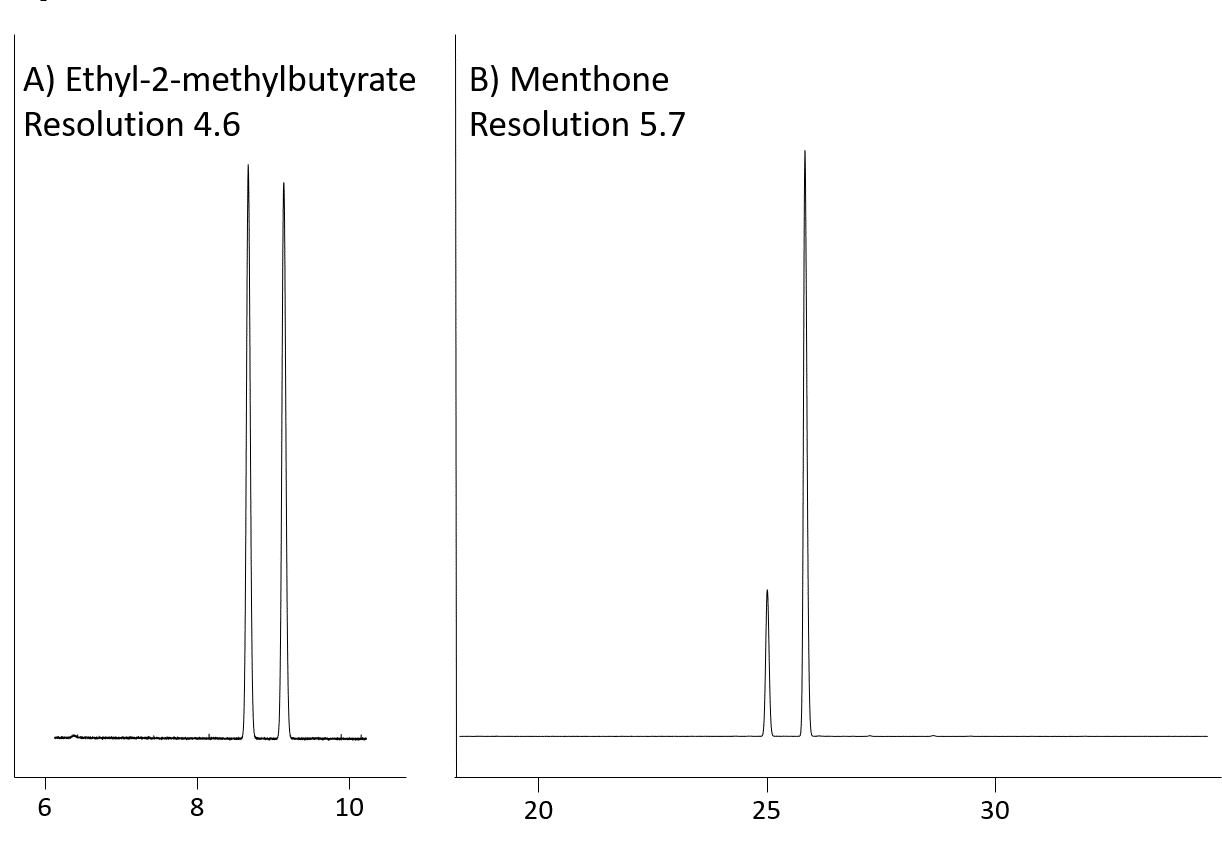
Figure 6: Limonene (A), camphor and styrene oxide (B) resolution on Rt-βDEXse (30m, 0.32mm ID, 0.25µm; cat. # 13106)



