
In our previous blogs we discussed solutions to reduce analysis time in situations where we had excessive resolution. Use of shorter columns, higher flow and faster programming allowed serious faster GC using existing instrumentation
Now we will move into a different situation and that is where we have “just enough resolution” to do the separation. Example chromatogram is shown in Fg.1. Any reduction in resolution will immediately affect the separation of the components. In this situation, we have only 2 options to choose from:
1: use hydrogen as the carrier gas;
2: use a shorter column that produce a higher efficiency;
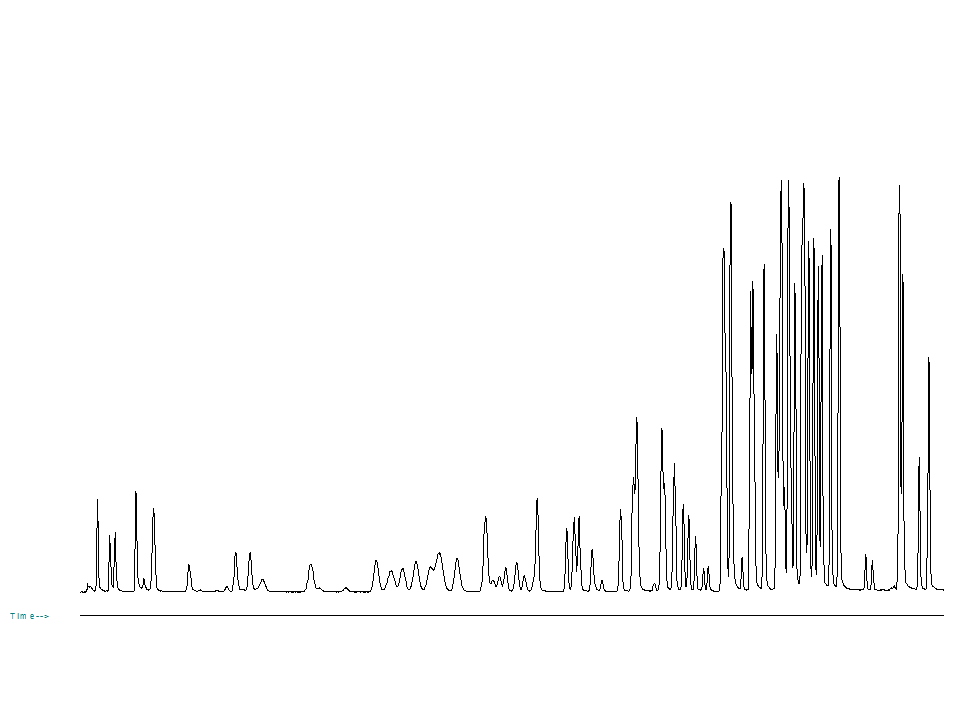
Fig. 1 Example of a complex chromatogram. Any loss in efficiency will immediately result in a separation challenge
The most easy way to reduce analysis time is to change the carrier gas. By using hydrogen instead of helium, we can benefit from the higher optimal linear velocity, which is a factor 2 higher, see fig. 2. At Restek, we test all our columns with hydrogen as the carrier gas, which saves us 50% on the investment in gas chromatographs.
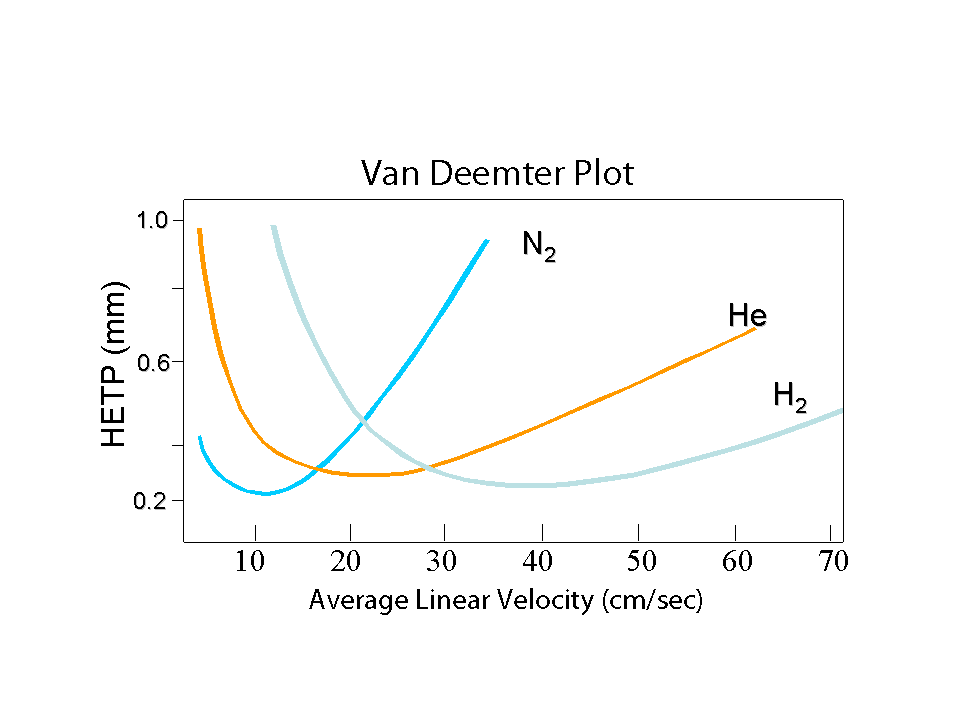
Fig. 2 Van Deemter curve for different gases. Hydrogen is 2x faster then helium
By changing to hydrogen we not only benefit form a faster analysis, we also benefit from a higher response. As the peaks elute 2x faster, the height will be 2 times higher. For a similar signal/noise, one can inject 50% of the sample, from which we can benefit in less contamination of liners and column inlet. Fig 3 shows an example. The pressures for using hydrogen are the same as we use with helium. Due to the lower hydrogen-viscosity, we get approximate the double linear velocity.
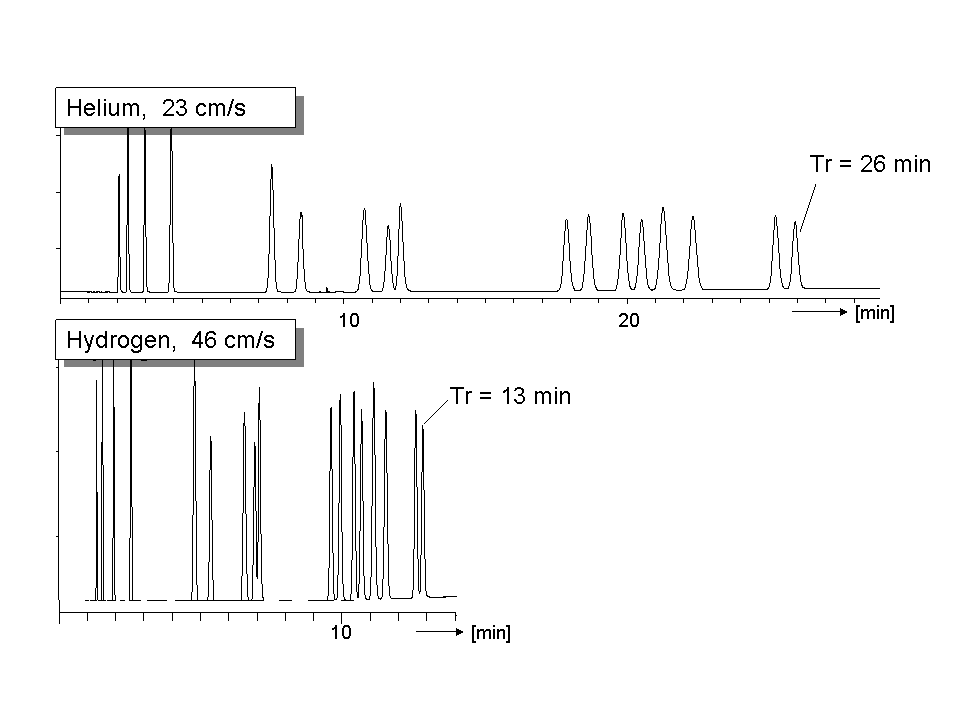
Fig. 3 Using hydrogen instead of helium does the same analysis in half the time and peaks are 2x higher..
For isothermal analysis the conversion is pretty straightforward. For temperature programmed analysis using hydrogen, we have to change the temperature program, to get the same elution temperatures, see fig.4. (this will give us also the factor 2 shorter run time and also makes sure that the peak elution order will not change). The change in temperature program is similar as we had to apply when we run the column at a higher gas velocity using the SAME carrier gas.(see: Part II: Impact of Higher Column Flow ). Only now, using hydrogen, we maintain the efficiency.
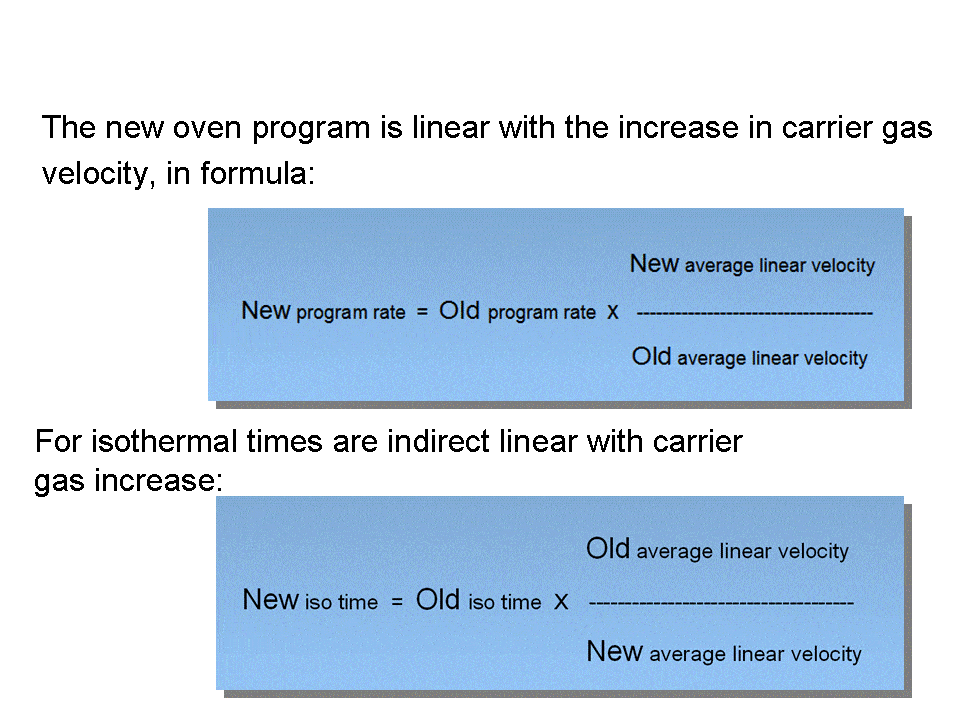
Fig.4 To get the same elution temperatures using hydrogen, we have to “calculate” the new oven program rate and the new Iso-times.
One of the biggest concerns is, that hydrogen is combustible. Indeed, it is combustable over a concentration range of 4% to 74% by volume, but we have to put this in perspective.
- The risk for building up these levels is reduced because of the enormous fast diffusion (dilution).
- Many labs already have a hydrogen gas line in place, used for fueling the FID. One can also use Hydrogen generators. These produce a relative small amount of hydrogen;
- The GC’s have nowadays digital flow controls. If one set a flow controlled carrier gas supply, it is impossible to have a large amount of hydrogen released in the oven. As soon as the column breaks, the pressure will be gone(its “flow” controlled), and the max. amount of hydrogen entering the oven is the injector volume and the actual hydrogen flow. It will be very difficult to even reach the 4% level;
- One can also buy hydrogen detection systems that you can install in the GCs. These systems use sensors that measure the air taken from the oven on hydrogen presence. If hydrogen is detected, the oven can be shut off and often an alternate gas can be turned on to protect the column.
- To reduce the risk to a minimum one can also use metal columns, like the MXT series You will be surprised how inert a metal column is. Example shown in figure 5 shows a 0.1 micron of a Rtx-5-coated MXT column. Test compounds are highly polar, as well as acidic and basic. All components elute with impressive peak symmetry. Restek developed and commercialized this series with a range of stationary phases.
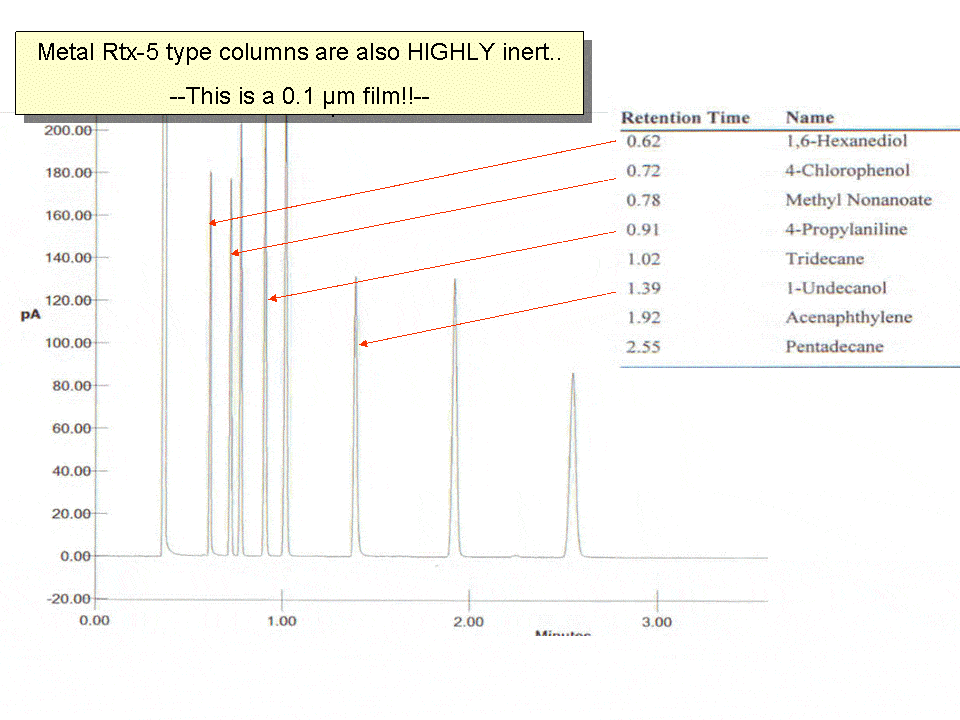
Fig. 5 Tough Polarity test mixture run on metal MXT column with only a 0.1 micron film.. Shows the unique quality of the Siltek deactivation
Hydrogen can be used with all injection systems. For safety reasons, the split vent is usually vented. In the early days when we used hydrogen. we always made a little “torch” on top of our GC’s and we just “lit” the hydrogen. As this is considered “open fire” it is not allowed in today’s labs.
The effects of using Hydrogen on detection systems is another concern. For most detection systems like FID, ECD, TCD, FPD, PFPD, SClD, I am not aware of issues. Of course, with FID and high column flows, one has to adjust the hydrogen feed of the detector. However for MS the use of hydrogen is not very clear, see also: Can I use Hydrogen as carrier gas when using Mass Spectrometric Detection?. Detectors like the NPD (the beads) do not seem to like hydrogen. Also PDD(HID) and ion traps need helium.
If you have any experiences using hydrogen, positive or negative, please share!



