Over the past several years, Δ8-THC has made headlines as a popular “legal” alternative to Δ9-THC. The rise in popularity of Δ8-THC led to a new analytical challenge for toxicology laboratories—differentiating between the Δ8 and Δ9 isomer forms. For labs performing this testing by LC-MS/MS, complete separation of these isomers and their metabolites is necessary for accurate quantitation. While HPLC stationary phases like Biphenyl and C18 have traditionally been used for toxicological analysis of cannabinoids, they lack the selectivity needed to adequately separate these isomers. The Raptor FluoroPhenyl column has emerged as a superior choice for analyzing THC isomers in biological matrices. The unique selectivity of this column allows for Δ8-THC, Δ9-THC, and their isomeric metabolites to be fully resolved. In the application note LC-MS/MS Analysis of THC Isomers & Metabolites in Whole Blood and Urine, we share complete LC-MS/MS workflows using this column for the analysis of both blood and urine matrices.
Recently, there have been quite a few new cannabinoids emerging on the market. These new compounds are often called semi-synthetic cannabinoids. This name comes from the fact that many of these compounds are found in low abundance in cannabis or hemp, so they are made synthetically and sold as naturally occurring, or their chemical structure is slightly modified to produce a new substance entirely. Earlier this year, The Center for Forensic Science Research & Education (CFSRE) added a semi-synthetic cannabinoids category to their quarterly scope recommendations, which highlights new and emerging drugs that laboratories should consider adding to their panels. As these compounds become more prominent, toxicology labs may look to add these new cannabinoids into to their Δ8/Δ9-THC analysis method.
Adding new compounds to a pre-existing method can be easier said than done, especially when isomers are involved. The method conditions may need to be adjusted to include the new compounds, while also ensuring the rest of the analytes in the method still perform as expected. In some cases, the addition of new analytes may necessitate extending the analytical runtime.
In the following work, we looked to add six semi-synthetic cannabinoids to an existing method that was developed for the analysis of Δ8-THC, Δ9-THC, and their hydroxy and carboxy metabolites. The method is described in the application note mentioned above for whole blood analysis. The six semi-synthetic cannabinoids (9(R)-HHC, 9(S)-HHC, Δ10-THC, THC-O-Acetate, THCP, and CBDP) were identified by the CFSRE as current scope recommendations. 9(R)-HHC and 9(S)-HHC are epimers, while THCP and CBDP are isomers. Δ10-THC is another isomer of Δ8-THC and Δ9-THC.
A sample containing 100 ng/mL of the analytes was run using the original method conditions. The method conditions and the resulting chromatogram are shown below.
Figure 1. Original method conditions and chromatogram including new semi-synthetic cannabinoids.
| Column | Raptor FluoroPhenyl 100 x 3 mm, 2.7 µm (cat.# 9319A1E) | ||
| Mobile Phase A | Water, 0.1% formic acid | ||
| Mobile Phase B | Methanol, 0.1% formic acid | ||
| Column Temperature | 40°C | ||
| Flow Rate | 0.8 mL/min | ||
| Gradient | Time (min) | %A | %B |
| 0.00 | 36 | 64 | |
| 6.50 | 36 | 64 | |
| 6.60 | 32 | 68 | |
| 13.00 | 32 | 68 | |
| 13.10 | 0 | 100 | |
| 14.00 | 0 | 100 | |
| 14.10 | 36 | 64 | |
| 16.00 | 36 | 64 | |
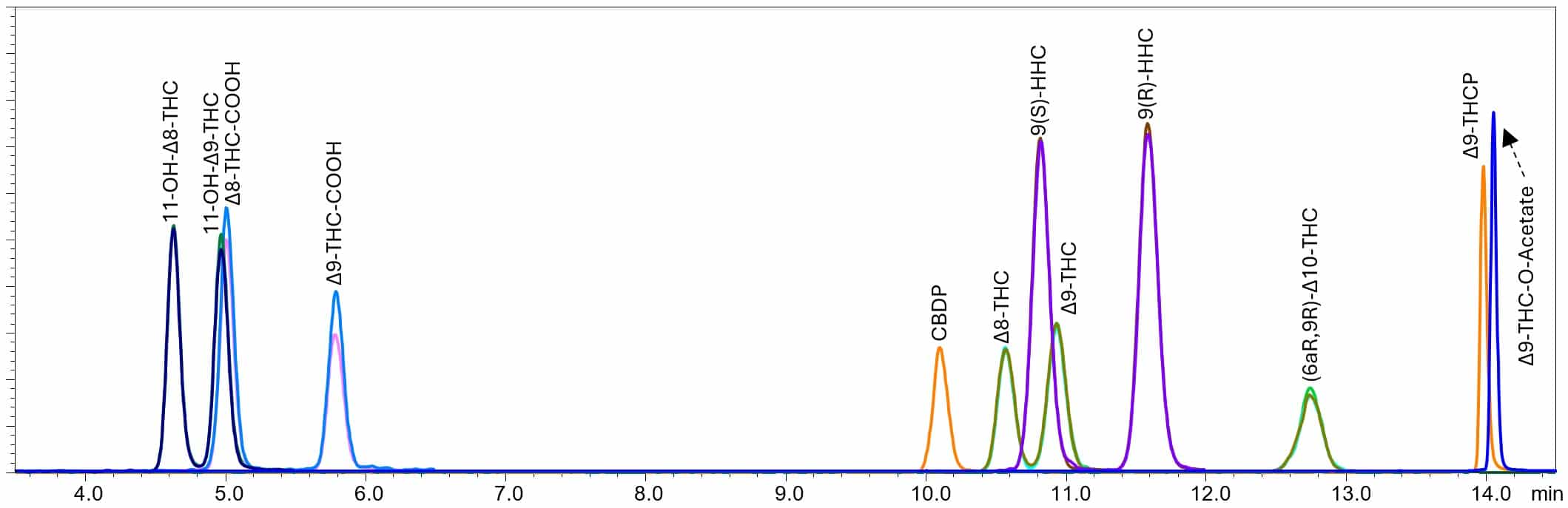
When analyzed using the original method conditions, all the critical pairs are resolved. However, THCP and THC-O-Acetate are highly retained and do not elute until around 14 minutes, while the gradient is ramped up to 100% mobile phase B. A hold at 100% organic is often implemented to elute or “wash” everything off the column, such as hydrophobic or very lipophilic components from the matrix. This step helps to reduce carryover in the next sample. It is not ideal to have analytes eluting in this area, as hydrophobic matrix components can co-elute and cause interference. The method parameters were adjusted to improve the performance of these analytes, while still maintaining separation of all critical pairs. The adjusted method conditions and resulting chromatogram are shown below.
Figure 2. Adjusted method conditions and chromatogram.
| Column | Raptor FluoroPhenyl 100 x 3 mm, 2.7 µm (cat.# 9319A1E) | ||
| Mobile Phase A | Water, 0.1% formic acid | ||
| Mobile Phase B | Methanol, 0.1% formic acid | ||
| Column Temperature | 30°C | ||
| Flow Rate | 0.8 mL/min | ||
| Gradient | Time (min) | %A | %B |
| 0.00 | 34 | 66 | |
| 5.50 | 34 | 66 | |
| 5.60 | 30 | 70 | |
| 10.50 | 30 | 70 | |
| 11.00 | 15 | 85 | |
| 13.00 | 0 | 100 | |
| 14.00 | 0 | 100 | |
| 14.10 | 34 | 66 | |
| 16.00 | 34 | 66 | |
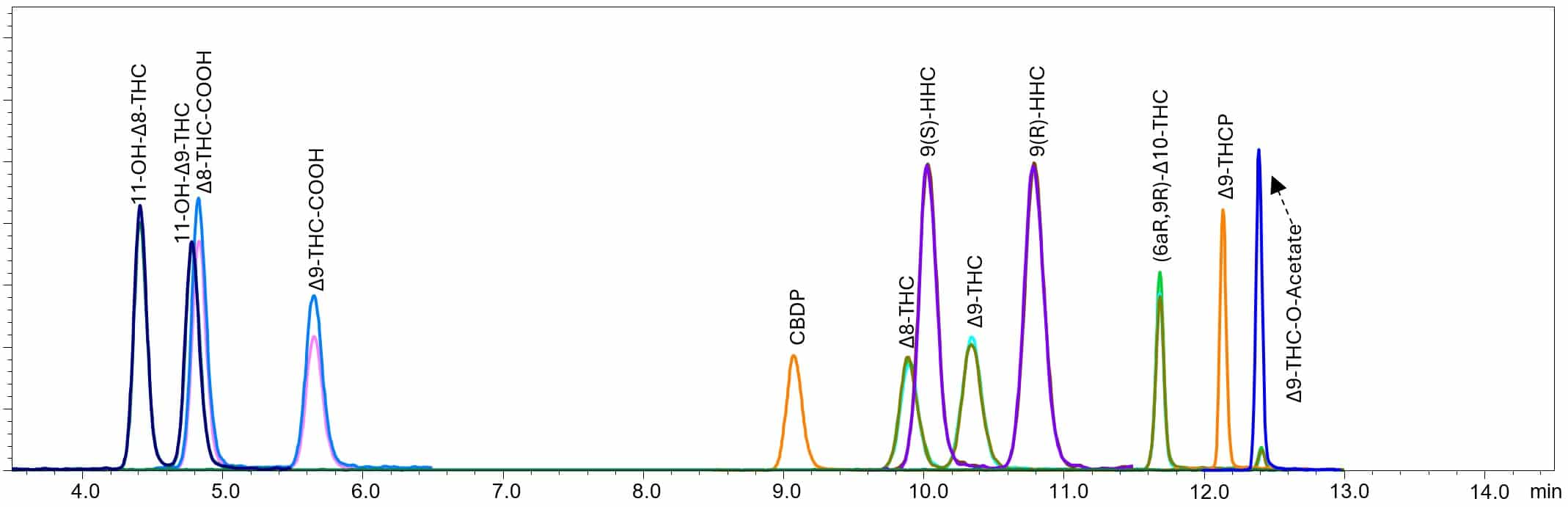
When analyzed using the adjusted method conditions, THCP and THC-O-Acetate elute at an earlier retention time, before the hold step at 100% B. All critical pairs are still resolved. This was achieved by lowering the column temperature (30°C vs. 40°C), which improved separation between the critical pairs, and altering the elution gradient. All six semi-synthetic cannabinoids were successfully incorporated into a method for Δ8/9-THC and metabolites, without having to extend the runtime.



