Welcome back my fragrance fanatics! Today, we continue exploring approaches for analyzing terpenes. Last time, we discussed two HS approaches that just flat out stunk! So, it’s time to make some adjusts. The first thing to check was the way our samples were prepared. We added 1 mL of our terpene standard to a 20 mL HS vial and tested it. This time, we will add some water and salt [sodium chloride (NaCl)] to help drive our less volatile terpenes (sesquiterpenes) into the gas phase. The idea for adding water is to help partition the terpenes into the gas phase described in USP 467. The sample preparation was 1 mL of terpene standard, 4 mL of DI water, and ~30% wt/wt NaCl, with a final terpene concentration of 1 µg/mL. We kept most of our parameters the same (previous blog link). However, we did drop the HS-Syringe incubation temperature from 140°C to 80°C in an effort to prevent excessive water vapor from moving into the gas phase. As you can see from the results below, we made some great improvements; mainly for the HS-SPME Arrow approach.
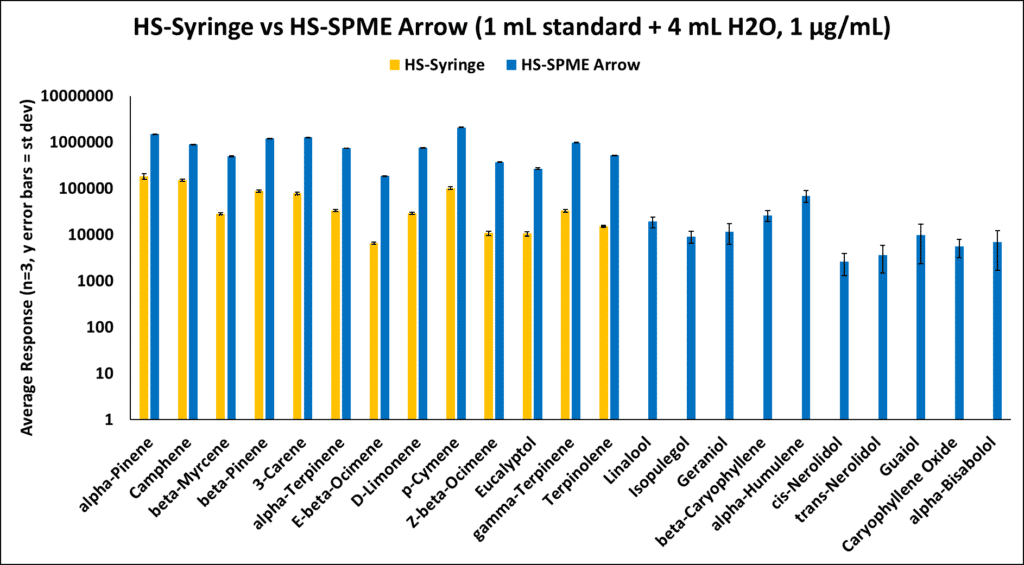
The addition of water and salt helped drive the less volatile terpenes into the gas phase. Unfortunately, they were unable to be captured by the HS-Syringe, but the HS-SPME Arrow did an excellent job adsorbing the terpenes. The HS-Syringe method shows low recoveries most likely because the higher molecular weight terpenes are condensing in the needle and not reaching the analytical column. Using the HS-SPME method, the compounds have good affinity for the fiber phase and remain in the fiber until they are desorbed into the inlet. So, now we are able to identify all 23 terpenes of interest! Are we done? Absolutely not! We believe this can still be improved. Stay tuned for our next blog!



