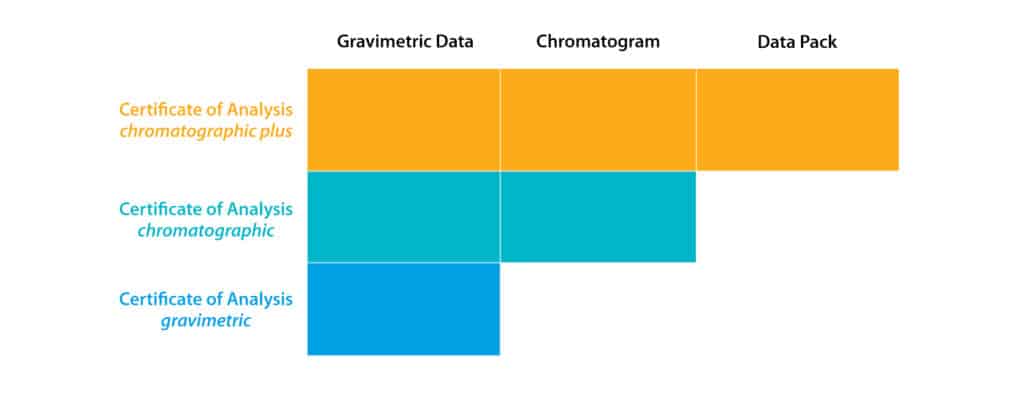
Restek offers three different types of Certificate of Analysis: gravimetric, chromatographic, and chromatographic plus. Each provides different pieces of documentation, which are outlined in the graphic below:
Nearly all our stock reference standards are provided with the highest level of documentation available—Certificate of Analysis (Chromatographic Plus)—and we provide all three options for most of our custom reference standards. Reference standards that fall within our ISO scopes of accreditation will be classified as certified reference materials (CRMs) regardless of documentation level.
- Certificate of Analysis gravimetric includes all raw material information, lot numbers and purity, isomer ratios for isomeric compounds, and calculated concentration(s). Associated uncertainties are also included for Certified Reference Materials (CRMs).
- Certificate of Analysis chromatographic includes all raw material information, lot numbers and purity, isomer ratios for isomeric compounds, and calculated concentration(s). Associated uncertainties are also included for Certified Reference Materials (CRMs). Additionally, a single sample is withdrawn from the packaged units and is tested via an appropriate technique to verify mixture composition. A chromatogram of the analyzed standard, with each peak identified, is included on the certificate.
- Certificate of Analysis chromatographic plus includes all raw material information, lot numbers and purity, isomer ratios for isomeric compounds, and calculated concentration(s). Associated uncertainties are also included for Certified Reference Materials (CRMs). Additionally, a sample of the packaged units is analyzed in triplicate and the peak areas are statistically compared to a second lot—either a previous lot (if available) or a freshly produced independent second lot. A chromatogram of the analyzed standard, with each peak identified, is included on the certificate. Plus, a detailed data pack containing the product certificate, all quantitative assay data and statistics, and the Reference Material Batch Record are available at www.restek.com/documentation
For more information, see GNBR1845.pdf (below).

