- Ensure crucial isomer separations of synthetic cathinones that are not easily separated with conventional C18 columns.
- Overcomes the challenge of not being able to fully separate and quantify isobaric isomers by MS/MS.
- Unique stationary phase provides good retention of aromatic compounds, resulting in sharper peak shapes.
In the last decade, synthetic cathinones have become widespread in recreational drug use, prompting a need for forensic toxicology laboratories to develop reliable workflows for their identification and quantification. Synthetic cathinones are derivatives of the naturally occurring compound cathinone and are a unique class of psycho-stimulants. They have an aromatic structure and contain structurally similar isomers that are challenging to separate using conventional C18 columns. A reliable analysis that achieves isomer separation is crucial because the pharmacological and toxicological properties of the various isomers differ significantly. Since the respective ortho, meta, and para isomers of methylmethcathinone (MMC) and methylethcathinone (MEC) are isobaric, they are not easily separated and quantified by MS/MS.
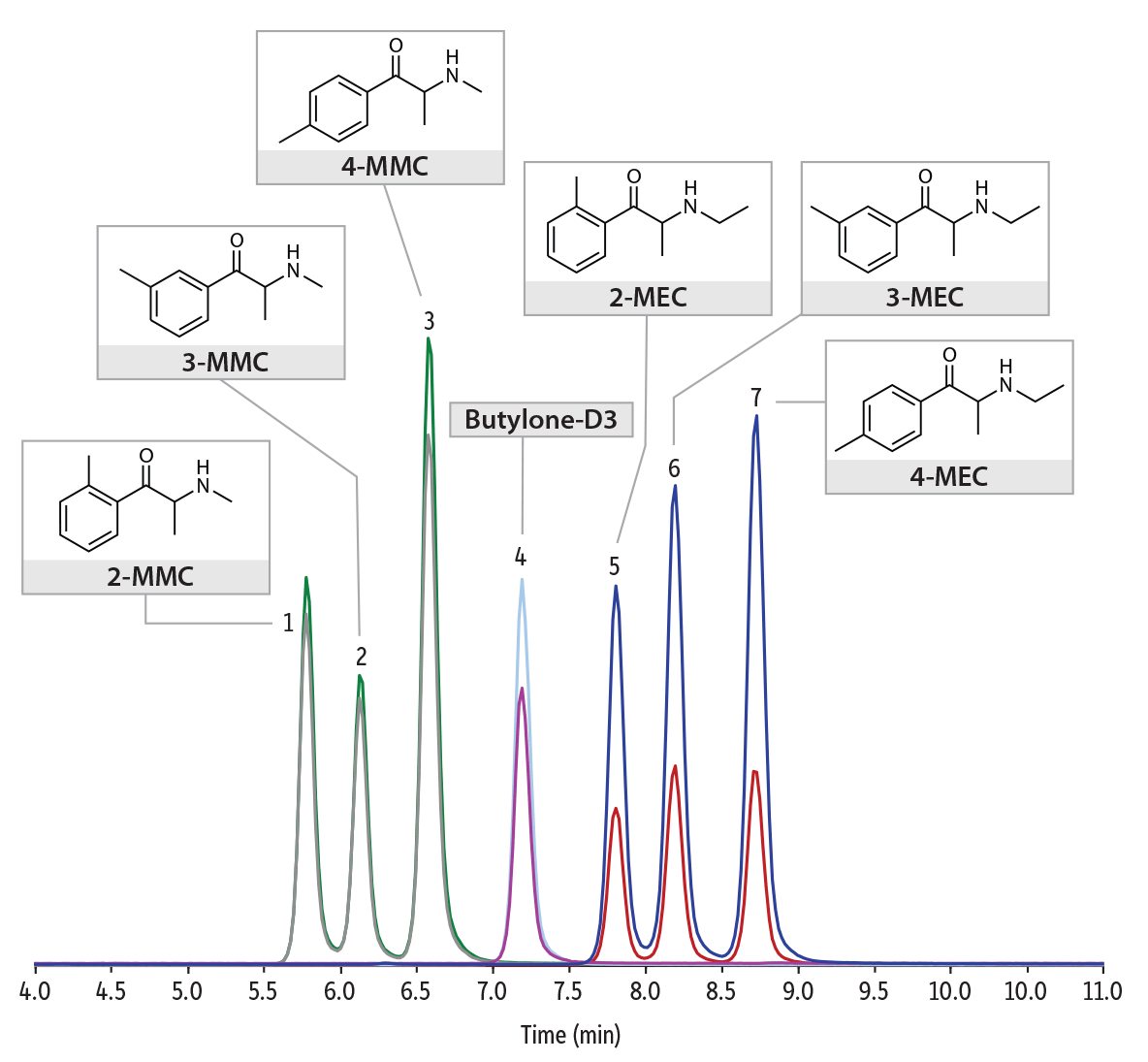
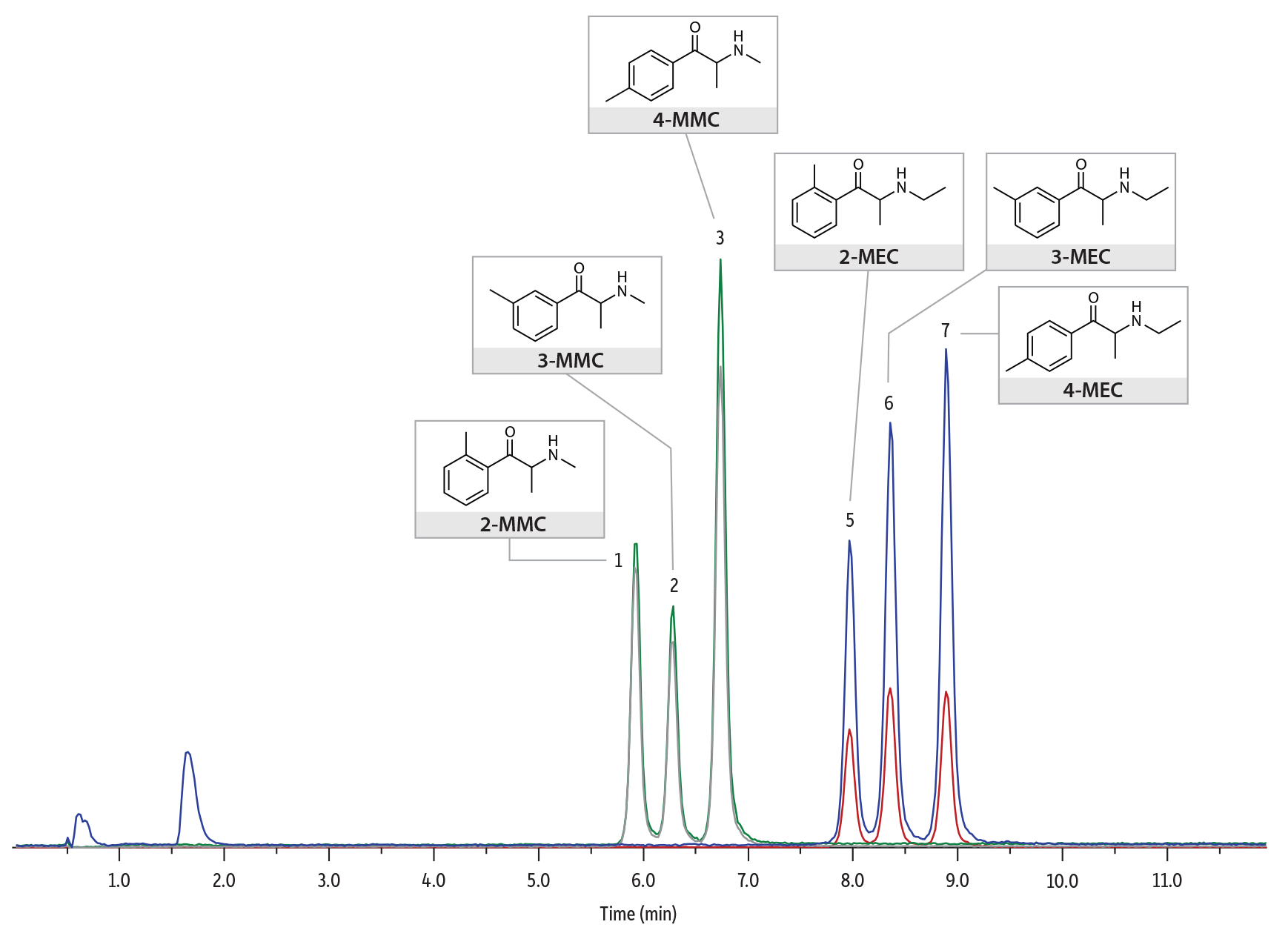
Here, we show the analysis of synthetic cathinone in serum samples on the Raptor Biphenyl core-shell column by LC-MS/MS analysis. The phase of this Raptor column exhibits strong pi-interactions, making it especially selective to the separation of the differently substituted aromatic rings that make up these isomers. A deuterated internal standard (butylone-d3) was chosen for use due to its availability and cost savings. Using the method conditions specified below, accurate quantitative results and reliable separations were achieved for the synthetic cathinone isomers of MMC and MEC, in serum samples. Figure 1 shows a section of the chromatogram from a spiked serum sample at 100 ng/mL to illustrate the isomer separations. Figure 2 shows a spiked serum sample at the limit of quantification, 5 ng/mL.
Figure 1: A spiked serum sample at 100 ng/mL shows excellent isomer separation.
LC_CF0790
Peaks
| Peaks | tR (min) | Parent Ion | Product Ion 1 | Product Ion 2 |
|---|
| 1. | 2-MMC | 5.75 | 178.1 | 145.1 | 160.0 |
| 2. | 3-MMC | 6.15 | 178.1 | 145.1 | 160.0 |
| 3. | 4-MMC | 6.70 | 178.1 | 145.1 | 160.0 |
| 4. | Butylone-D3 | 7.25 | 225.1 | 176.9 | |
| 5. | 2-MEC | 7.77 | 192.1 | 174.0 | 144.0 |
| 6. | 3-MEC | 8.30 | 192.1 | 174.0 | 144.0 |
| 7. | 4-MEC | 8.71 | 192.1 | 174.0 | 144.0 |
Conditions
| Raptor Biphenyl (cat.# 9309A12) |
| 100 mm x 2.1 mm ID |
| 2.7 µm |
| 90 Å |
|
| 50 °C |
| Water |
| 100 ng/mL |
| | 10 µL |
| Water:methanol 95:5, 0.1% formic acid |
| Methanol, 0.1% formic acid |
| | Time (min) | Flow (mL/min) | %A | %B |
|---|
| 0.00 | 0.5 | 95 | 5 | | 1.00 | 0.5 | 95 | 5 | | 12.00 | 0.5 | 83 | 17 | | 12.01 | 0.5 | 2 | 98 | | 20.00 | 0.5 | 2 | 98 | | 20.01 | 0.5 | 95 | 5 | | 23.00 | 0.5 | 95 | 5 |
|
| SCIEX API 4000 QTRAP MS |
| ESI+ |
Figure 2: Spike serum sample at the LOQ, 5 ng/mL.
LC_CF0789
Peaks
| Peaks | tR (min) | Parent Ion | Product Ion 1 | Product Ion 2 |
|---|
| 1. | 2-MMC | 5.95 | 178.1 | 145.1 | 160.0 |
| 2. | 3-MMC | 6.25 | 178.1 | 145.1 | 160.0 |
| 3. | 4-MMC | 6.76 | 178.1 | 145.1 | 160.0 |
| 4. | Butylone-D3 | 7.45 | 225.1 | 176.9 | – |
| 5. | 2-MEC | 7.99 | 192.1 | 174.0 | 144.0 |
| 6. | 3-MEC | 8.38 | 192.1 | 174.0 | 144.0 |
| 7. | 4-MEC | 8.90 | 192.1 | 174.0 | 144.0 |
Conditions
| Raptor Biphenyl (cat.# 9309A12) |
| 100 mm x 2.1 mm ID |
| 2.7 µm |
| 90 Å |
|
| 50 °C |
| Water |
| 5 ng/mL |
| | 10 µL |
| Water:methanol 95:5, 0.1% formic acid |
| Methanol, 0.1% formic acid |
| | Time (min) | Flow (mL/min) | %A | %B |
|---|
| 0.00 | 0.5 | 95 | 5 | | 1.00 | 0.5 | 95 | 5 | | 12.00 | 0.5 | 83 | 17 | | 12.01 | 0.5 | 2 | 98 | | 20.00 | 0.5 | 2 | 98 | | 20.01 | 0.5 | 95 | 5 | | 23.00 | 0.5 | 95 | 5 |
|
| SCIEX API 4000 QTRAP MS |
| ESI+ |
Acknowledgements:
Data courtesy of Alexandra Maas, Ph.D., University of Bonn. Thank you to Mrs. Maas for allowing us to use this data.
References:
A. Maas, et al., Separation of ortho, meta and para isomers of methylmethcathinone (MMC) and methylethcathinone (MEC) using LC-ESI-MS/MS: Application to forensic serum samples, J. Chromatogr. B (2017), http://dx.doi.org/10.1016/j.jchromb.2017.01.046
-
Restek is a leading provider of chromatography columns, accessories, and certified reference materials. Trust Restek for reliable, high-quality analytical solutions.
View all posts
PHFA4018