For some years now, the development departments of high-pressure liquid chromatography companies have been dealing with the question of what influence the surfaces of the instrument and component hardware can have on peak shape and sensitivity in the detection of polar, chelating molecules. Biomolecules in particular, with their complex acid/base properties, but also compounds such as glyphosate and AMPA, with their organophosphate groups, show that the surface properties of the components in the sample path have a decisive influence on peak shape and sensitivity.
Recently, Restek developed a inert LC column line to address this issue more efficiently and effectively than short-term solutions, such as priming or medronic acid deactivation. Reports from several independent laboratories indicate that these new columns can offer substantial benefits for pesticides and mycotoxins analysis.
In June 2024, Maria Antonietta Carrera from the Department of Desertification and Geo-ecology, Experimental Station of Arid Zones in Almeria, under the supervision of Amadeo Fernandez-Alba from the European Union Reference Laboratory for Pesticide Residues in Fruit & Vegetables (EURL-FV), published a remarkable paper on Simultaneous analysis of pesticides and mycotoxins in primary processed foods: The case of bee pollen”, in which she compared a column commonly used for routine analysis of the analytes under consideration with a Raptor Inert ARC-18 column from Restek.
In her publication she describes “As reported in Fig. 3, no substantial differences were revealed by this test, since almost the same number of compounds could be detected at a concentration of 1 μg/kg with both columns. However, by looking at peaks obtained for about 15 % of analytes at 1 μg/kg, it was possible to notice a visible improvement in the peak shape (Fig. 4) when using the Raptor Inert column. This particular feature can be sometimes decisive for the identification of the analytes in real samples.”
Please find the full publication here: https://www.cell.com/heliyon/fulltext/S2405-8440(24)09543-4
In addition to influential labs, such as EURL, that assess the application of new analytical developments within their specific fields, method developers in instrument companies must ensure that the performance statement about their devices is not countered by poor chromatography, for which they bear no responsibility.
LCTech GmbH has made a name for itself in automated sample preparation for the analysis of mycotoxins, PFAS, and other multicomponent analyte panels. Frederik Wuppermann, Product Manager Biotechnology at LCTech, describes his experience with the inert columns in mycotoxin analysis as follows.
“The separation of aflatoxin B1, G1, B2, G2, OTA, zearalenone, deoxynivalenol, T2, H-T2, fumonisin B1 and B2 after CrossTOX cleanup in high-throughput mycotoxin analysis was very good. The shorter conditioning times were also very beneficial. I can certainly also imagine the Inert Biphenyl column as an alternative for other applications.”
In addition to these independent reports, Restek has assessed the impact of the new inert LC columns on pesticides and mycotoxins. We selected these groups of analytes because they include a variety of diverse compound chemistries and are often analyzed together in multi-class screening analyses. In such screening analyses, it is very important to ensure reliable identification and quantification of the individual components at trace levels (low LOQs), and an inert LC column can help with this by providing proper peak shape and good sensitivity. For both groups, we selected an appropriate stationary phase, analyzed the target compounds on both conventional LC columns and the new inert LC columns, and compared the chromatographic results. Here are our findings.
Pesticides
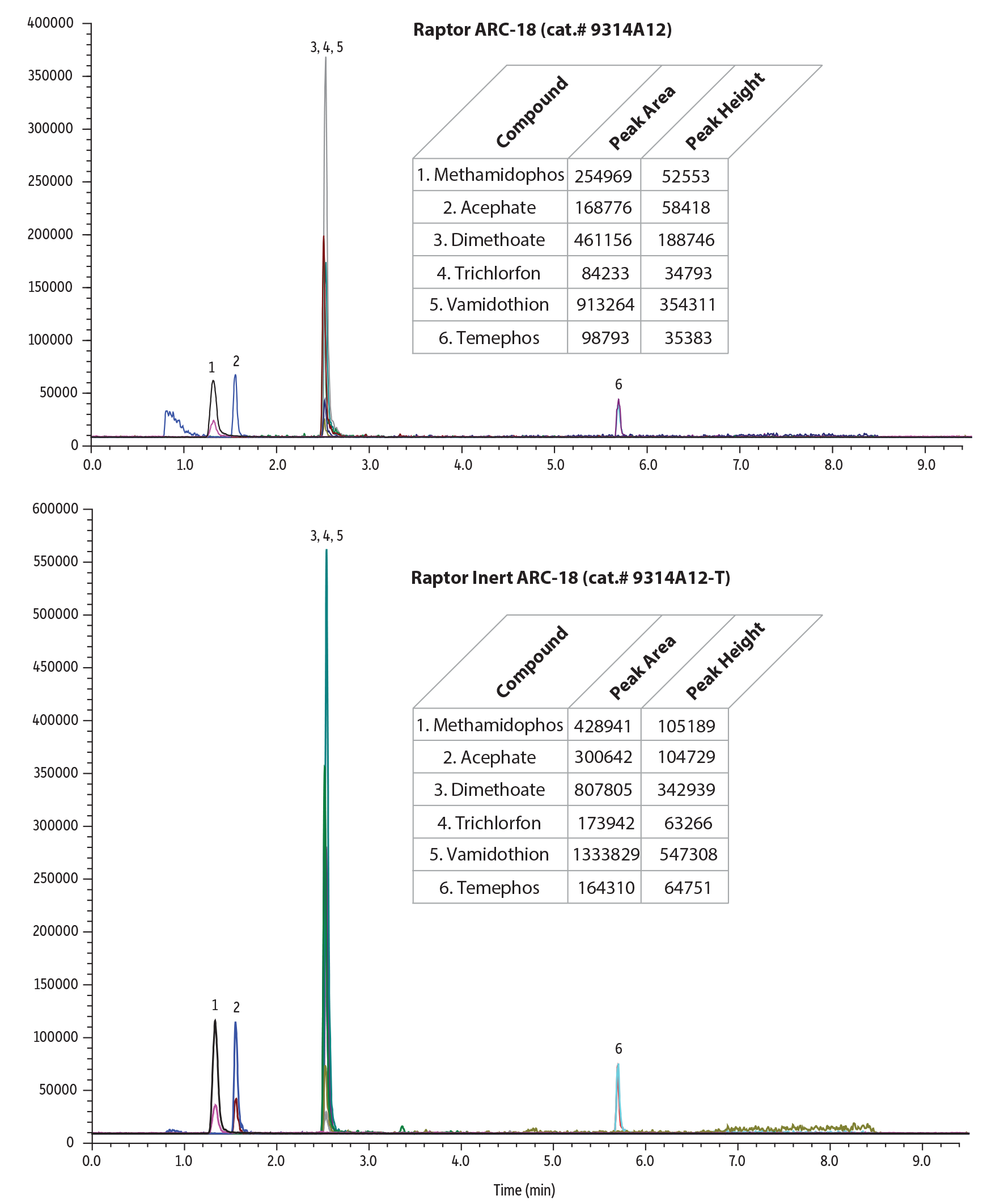
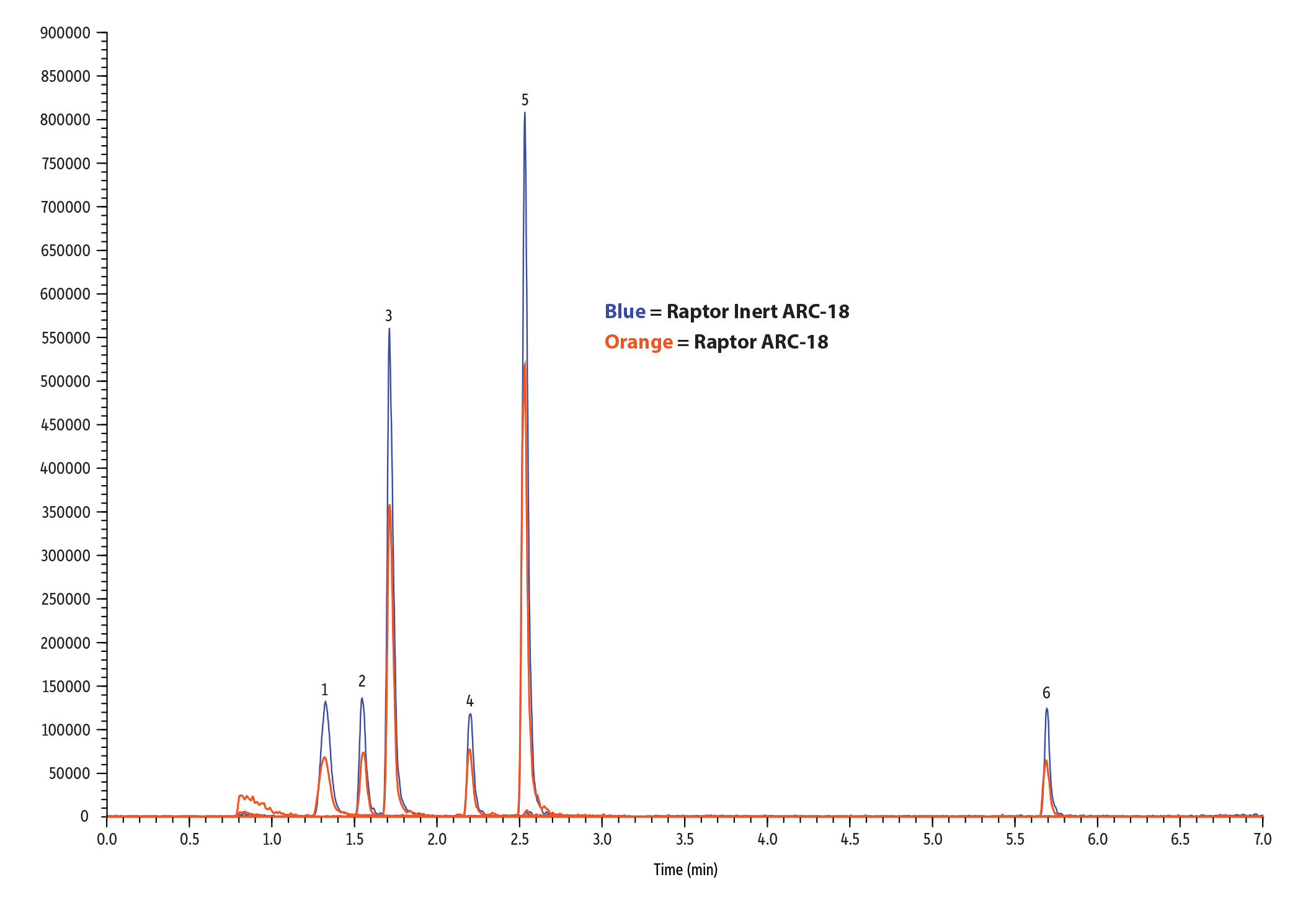
For this experiment, we compared a Raptor Inert ARC-18 column to a standard Raptor ARC-18 column. Pesticides analysis can be challenging because these panels typically contain a wide variety of compound chemistries. Phosphorylated, acidic, polar compounds, and/or metal chelating species, such as organophosphate pesticides, can react to the metal surfaces inside standard, stainless-steel analytical columns, which can degrade chromatographic performance. In contrast, the deactivated hardware in Restek’s inert LC columns significantly improved chromatographic results (Figures 1-2, Table I).
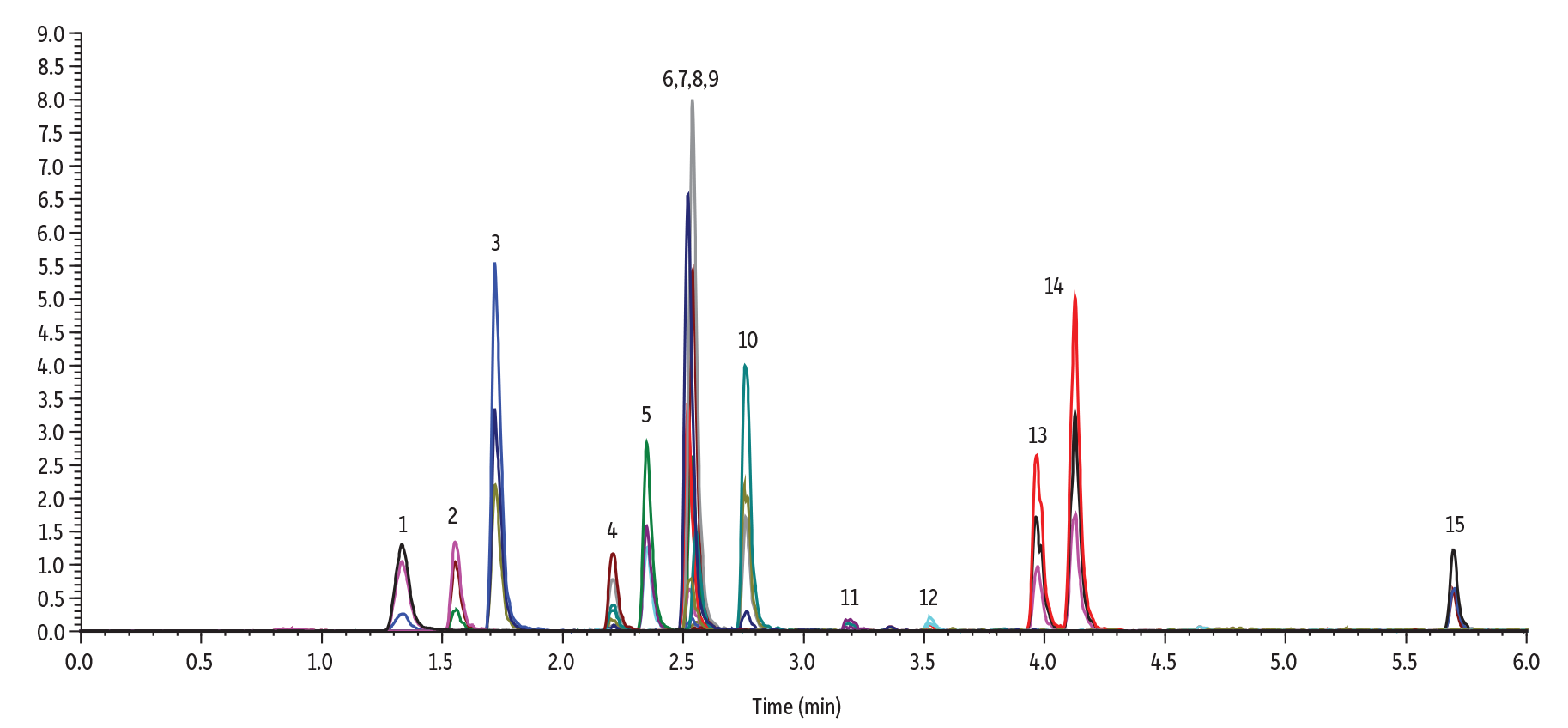
LC_EV0596
Peaks
| Peaks | tR (min) | Precursor Ion | Product Ion 1 | Product Ion 2 | Peak Area | Peak Height | |
|---|---|---|---|---|---|---|---|
| 1. | Methamidophos | 1.33 | 142.0 | 94.0 | 125.1 | 428941 | 105189 |
| 2. | Acephate | 1.55 | 184.0 | 143.0 | 48.9 | 300642 | 104729 |
| 3. | Omethoate | 1.72 | 214.0 | 125.0 | 182.9 | 892008 | 337690 |
| 4. | Monocrotophos | 2.21 | 224.1 | 127.0 | 193.1 | 215810 | 78425 |
| 5. | Dicrotophos | 2.35 | 238.1 | 112.1 | 72.0 | 404916 | 159292 |
| 6. | Dimethoate | 2.52 | 230.0 | 125.0 | 199.0 | 807805 | 342939 |
| 7. | Trichlorfon | 2.53 | 257.0 | 108.9 | 220.8 | 173942 | 63266 |
| 8. | Vamidothion | 2.54 | 288.0 | 146.0 | 118.0 | 1333829 | 547308 |
| 9. | Mevinphos isomer 1 | 2.55 | 241.9 | 126.9 | 192.9 | 311274 | 129961 |
| 10. | Mevinphos isomer 2 | 2.76 | 241.9 | 126.9 | 192.9 | 74030 | 29802 |
| 11. | Carbaryl | 3.18 | 202.1 | 145.0 | 127.0 | 39671 | 11924 |
| 12. | Isocarbophos | 3.52 | 291.1 | 231.1 | 121.1 | 33294 | 11941 |
| 13. | Dimethomorph isomer 1 | 3.96 | 388.2 | 300.9 | 165.1 | 511766 | 172977 |
| 14. | Dimethomorph isomer 2 | 4.13 | 388.2 | 300.9 | 165.1 | 877031 | 328826 |
| 15. | Temephos | 5.70 | 467.1 | 124.9 | 418.9 | 164310 | 64751 |
Conditions
| Column | Raptor Inert ARC-18 (cat.# 9314A12-T) | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||||||||||
| Temp.: | 50 °C | ||||||||||||||||||||||||||||||||
| Standard/Sample | LC multiresidue pesticide standard #1 (cat.# 31972) | ||||||||||||||||||||||||||||||||
| Diluent: | Water, 0.1% formic acid | ||||||||||||||||||||||||||||||||
| Conc.: | 1 ng/mL | ||||||||||||||||||||||||||||||||
| Inj. Vol.: | 5 µL | ||||||||||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||||||||||
| A: | Water, 2 mM ammonium formate, 0.1% formic acid | ||||||||||||||||||||||||||||||||
| B: | Methanol, 2 mM ammonium formate, 0.1% formic acid | ||||||||||||||||||||||||||||||||
|
| Detector | Shimadzu LCMS-8060 |
|---|---|
| Ion Mode: | ESI+ |
| Mode: | MRM |
| Instrument | Shimadzu Nexera X2 |
Table I: Pesticide peak areas and heights were up to 2X higher on Raptor Inert LC columns compared to standard columns.
| Compound | Peak Area | Peak Height | ||||
| Stainless Steel | Inert | Areas Ratio (Inert/Stainless Steel) | Stainless Steel | Inert | Height Ratio (Inert/Stainless Steel) | |
| Methamidophos | 254969 | 428941 | 1.68 | 52553 | 105189 | 2.00 |
| Acephate | 168776 | 300642 | 1.78 | 58418 | 104729 | 1.79 |
| Omethoate | 579502 | 892008 | 1.54 | 216157 | 337690 | 1.56 |
| Monocrotophos | 140095 | 215810 | 1.54 | 51402 | 78425 | 1.53 |
| Dicrotophos | 340978 | 404916 | 1.19 | 135380 | 159292 | 1.18 |
| Dimethoate | 461156 | 807805 | 1.75 | 188746 | 342939 | 1.82 |
| Trichlorfon | 84233 | 173942 | 2.07 | 34793 | 63266 | 1.82 |
| Vamidothion | 913264 | 1333829 | 1.46 | 354311 | 547308 | 1.54 |
| Mevinphos isomer 1 | 213632 | 311274 | 1.46 | 82105 | 129961 | 1.58 |
| Mevinphos isomer 2 | 56093 | 74030 | 1.32 | 29070 | 29802 | 1.03 |
| Carbaryl | 43590 | 39671 | 0.91 | 14563 | 11924 | 0.82 |
| Isocarbophos | 21587 | 33294 | 1.54 | 9062 | 11941 | 1.32 |
| Dimethomorph isomer 1 | 462425 | 511766 | 1.11 | 166990 | 172977 | 1.04 |
| Dimethomorph isomer 2 | 896109 | 877031 | 0.98 | 311657 | 328826 | 1.06 |
| Temephos | 98793 | 164310 | 1.66 | 35383 | 64751 | 1.83 |
Mycotoxins
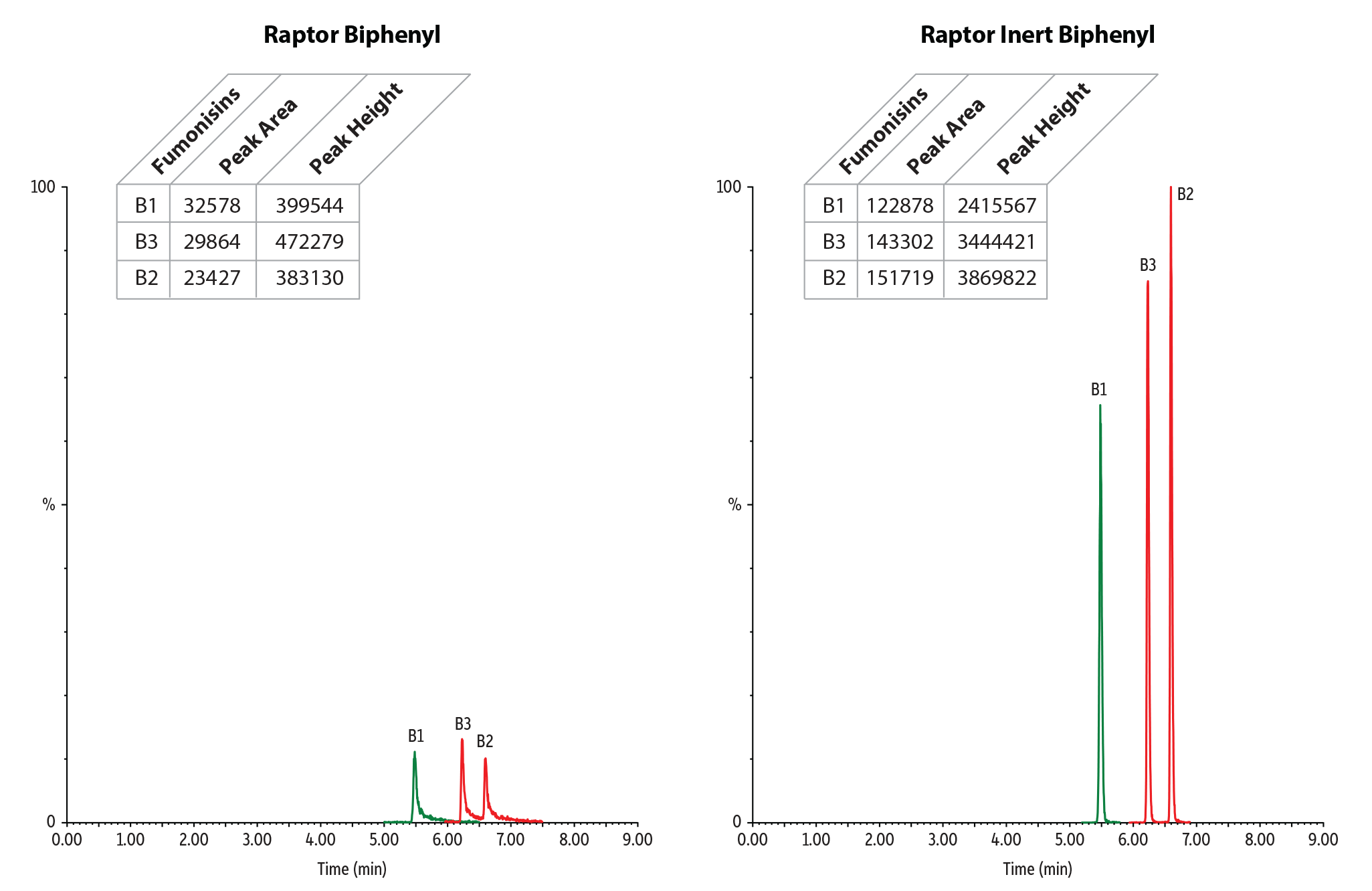
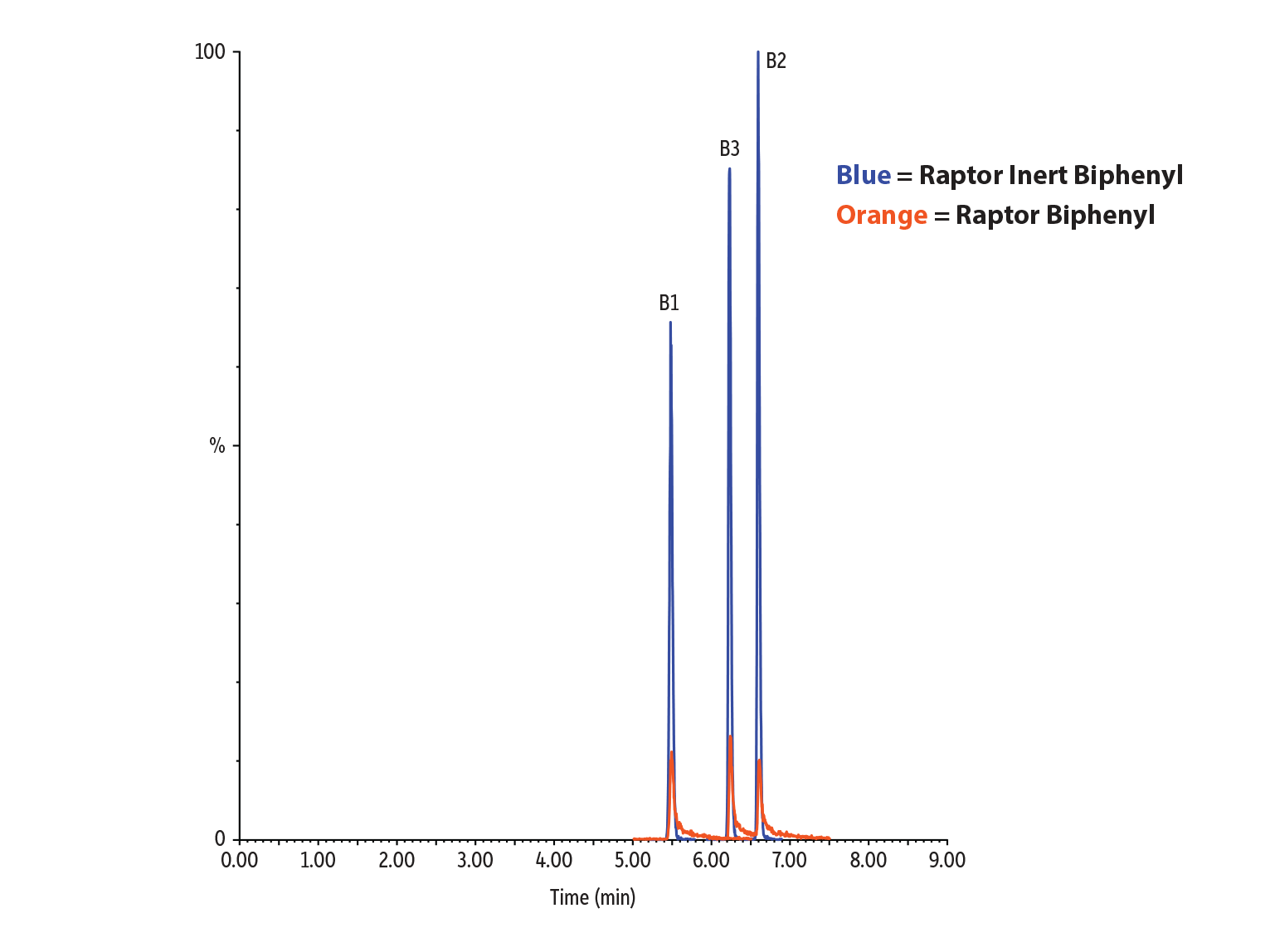
In addition to pesticides analysis, we also wanted to test whether using an inert LC column could improve results for mycotoxins analysis. Because mycotoxins are reactive compounds that can have acidic, polar, or metal-chelating groups, extensive column conditioning and equilibration is often required to obtain adequate chromatography. For these experiments, we used columns with a Biphenyl stationary phase for optimal selectivity. The results shown in Figures 3-4 and Table II clearly demonstrate that peak shape and response for mycotoxins can also be improved by using an inert column.
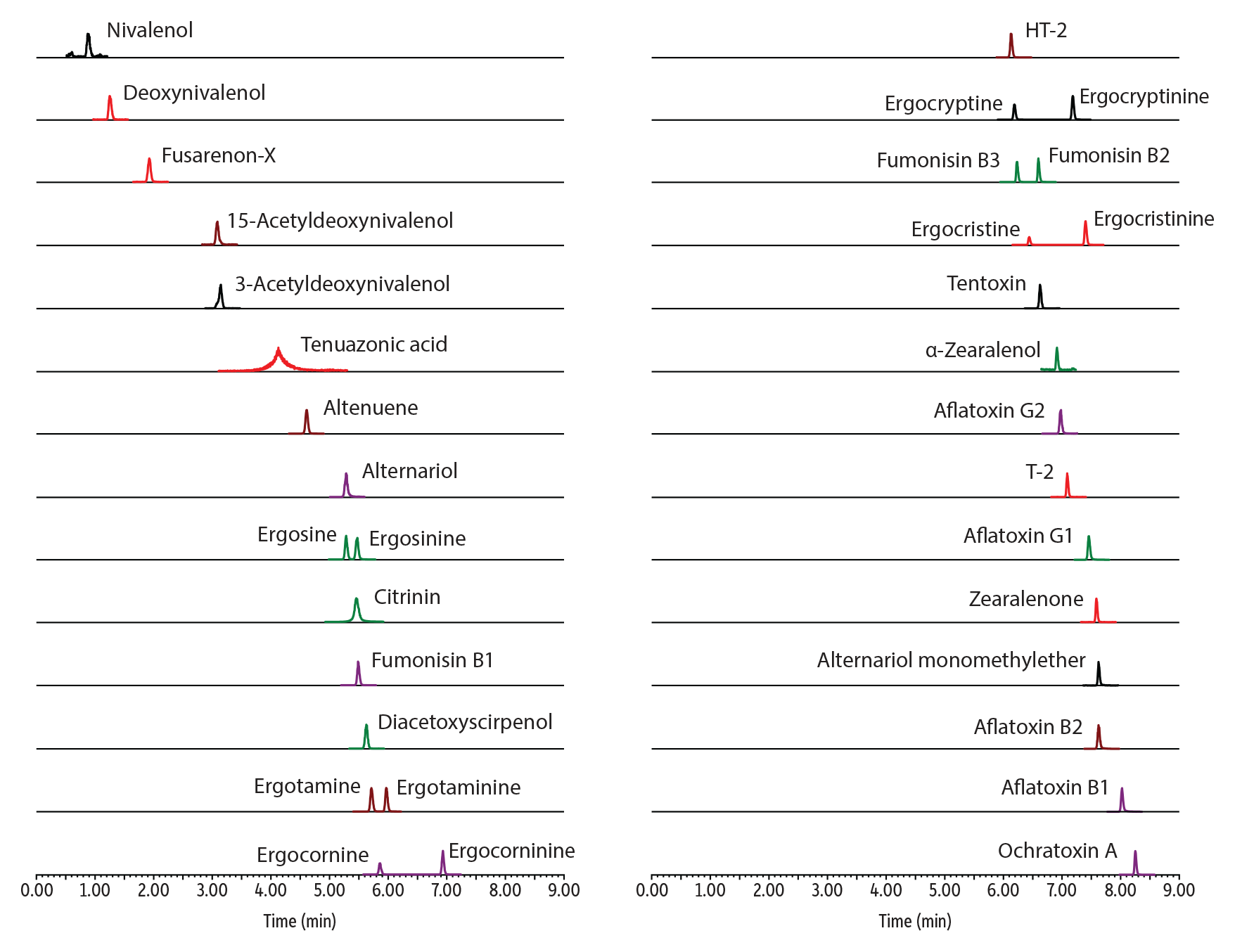
LC_FS0552
Peaks
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Peak Area | Peak Height | |
|---|---|---|---|---|---|---|---|
| 1. | Nivalenol | 0.88 | 10 | 295.1 | 137.1 | 4182 | 64495 |
| 2. | Deoxynivalenol | 1.25 | 10 | 297.2 | 231.0 | 17346 | 281906 |
| 3. | Fusarenon-X | 1.92 | 10 | 355.1 | 137.1 | 7668 | 121790 |
| 4. | 15-Acetyldeoxynivalenol | 3.08 | 10 | 339.2 | 137.1 | 31369 | 517570 |
| 5. | 3-Acetyldeoxynivalenol | 3.14 | 10 | 339.2 | 213.1 | 22613 | 296396 |
| 6. | Tenuazonic acid | 4.11 | 10 | 198.1 | 125.0 | 47828 | 197658 |
| 7. | Altenuene | 4.60 | 10 | 293.2 | 257.1 | 113850 | 2059699 |
| 8. | Alternariol | 5.27 | 10 | 259.0 | 185.1 | 73272 | 1302192 |
| 9. | Ergosine | 5.28 | 10 | 548.4 | 208.1 | 486620 | 9366601 |
| 10. | Citrinin | 5.46 | 10 | 251.2 | 233.1 | 1007880 | 9828889 |
| 11. | Ergosinine | 5.46 | 10 | 548.4 | 208.1 | 496734 | 8740527 |
| 12. | Fumonisin B1 | 5.48 | 10 | 722.5 | 352.3 | 122878 | 2415567 |
| 13. | Diacetoxyscirpenol | 5.62 | 10 | 384.2 | 247.1 | 68139 | 1208825 |
| 14. | Ergotamine | 5.71 | 10 | 582.4 | 223.2 | 493003 | 9274155 |
| 15. | Ergocornine | 5.85 | 10 | 562.4 | 268.2 | 387025 | 7732744 |
| 16. | Ergotaminine | 5.96 | 10 | 582.4 | 223.2 | 462119 | 9237991 |
| 17. | HT-2 | 6.13 | 10 | 447.2 | 345.1 | 15221 | 323765 |
| 18. | Ergocryptine | 6.19 | 10 | 576.4 | 268.2 | 522204 | 11360838 |
| 19. | Fumonisin B3 | 6.23 | 10 | 706.4 | 336.2 | 143302 | 3444421 |
| 20. | Ergocristine | 6.44 | 10 | 610.4 | 223.2 | 195562 | 4450058 |
| 21. | Fumonisin B2 | 6.59 | 10 | 706.4 | 336.2 | 151719 | 3869822 |
| 22. | Tentoxin | 6.62 | 10 | 415.2 | 312.2 | 95175 | 2131906 |
| 23. | α-Zearalenol | 6.91 | 10 | 303.1 | 285.1 | 30224 | 702420 |
| 24. | Ergocorninine | 6.93 | 10 | 562.4 | 268.2 | 704029 | 14389283 |
| 25. | Aflatoxin G2 | 6.97 | 10 | 331.2 | 189.0 | 262824 | 5274353 |
| 26. | T-2 | 7.09 | 10 | 489.2 | 387.1 | 56535 | 1394735 |
| 27. | Ergocryptinine | 7.18 | 10 | 576.4 | 268.2 | 778972 | 16765348 |
| 28. | Ergocristinine | 7.40 | 10 | 610.4 | 223.2 | 1583053 | 32975663 |
| 29. | Aflatoxin G1 | 7.45 | 10 | 329.1 | 199.7 | 304389 | 6102959 |
| 30. | Zearalenone | 7.59 | 10 | 319.2 | 283.1 | 37162 | 927455 |
| 31. | Alternariol monomethylether | 7.62 | 10 | 273.0 | 199.1 | 31024 | 640689 |
| 32. | Aflatoxin B2 | 7.63 | 10 | 315.1 | 287.0 | 295648 | 5724754 |
| 33. | Aflatoxin B1 | 8.02 | 10 | 313.2 | 241.1 | 223520 | 4425821 |
| 34. | Ochratoxin A | 8.25 | 10 | 404.1 | 239.0 | 190060 | 4411953 |
Conditions
| Column | Raptor Inert Biphenyl (cat.# 9309A12-T) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||
| Temp.: | 60 °C | ||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||
| Aflatoxins standard (cat.# 34121) | |||||||||||||||||||||||||
| Ochratoxin A standard (cat.# 34122) | |||||||||||||||||||||||||
| Diluent: | 50:50 Water:methanol | ||||||||||||||||||||||||
| Conc.: | 10 ng/mL | ||||||||||||||||||||||||
| Inj. Vol.: | 5 µL | ||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||
| A: | Water, 0.05% formic acid | ||||||||||||||||||||||||
| B: | Methanol, 0.05% formic acid | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Max Pressure: | 440 bar |
| Detector | Waters Xevo TQ-S |
|---|---|
| Ion Mode: | ESI+ |
| Mode: | MRM |
| Instrument | Waters ACQUITY UPLC I-Class |
| Notes |
Table II: For mycotoxins, peak height increased up to 10X and peak area increased up to 6X when using a Raptor Inert Biphenyl column compared to a standard column.
| Compound | Peak Area | Peak Height | ||||
| Stainless Steel | Inert | Areas Ratio (Inert/Stainless Steel) | Stainless Steel | Inert | Height Ratio (Inert/Stainless Steel) | |
| Fumonisin B1 | 32578 | 122878 | 3.77 | 399544 | 2415567 | 6.05 |
| Fumonisin B2 | 23427 | 151719 | 6.48 | 383130 | 3869822 | 10.10 |
| Fumonisin B3 | 29864 | 143302 | 4.80 | 472279 | 3444421 | 7.29 |
| Ergocristine | 171197 | 195562 | 1.14 | 3865898 | 4450058 | 1.15 |
| Ergocristinine | 1393116 | 1583053 | 1.14 | 29212317 | 32975663 | 1.13 |
| Ergotamine | 433635 | 493003 | 1.14 | 8149518 | 9274156 | 1.14 |
| Ergotaminine | 397370 | 462119 | 1.16 | 7885403 | 9237991 | 1.17 |
| Ergocryptine | 446481 | 522204 | 1.17 | 9671753 | 11360839 | 1.17 |
| Ergocryptinine | 658788 | 778972 | 1.18 | 13680420 | 16765348 | 1.23 |
| Ergocornine | 370509 | 387025 | 1.04 | 7248981 | 7732744 | 1.07 |
| Ergocorninine | 590167 | 704029 | 1.19 | 12052359 | 14389283 | 1.19 |
| Ergosine | 445243 | 486620 | 1.09 | 8630932 | 9366602 | 1.09 |
| Ergosinine | 439026 | 496734 | 1.13 | 7820785 | 8740527 | 1.12 |
| T-2 | 43286 | 56535 | 1.31 | 1046233 | 1394735 | 1.33 |
| HT-2 | 10183 | 15221 | 1.49 | 216703 | 323765 | 1.49 |
| Tentoxin | 70973 | 95175 | 1.34 | 1577164 | 2131907 | 1.35 |
| Ochratoxin | 173686 | 190060 | 1.09 | 4039682 | 4411953 | 1.09 |
| Diacetoxyscirpenol | 47850 | 68139 | 1.42 | 846403 | 1208826 | 1.43 |
| Fusarenone X | 3865 | 7668 | 1.98 | 60409 | 121790 | 2.02 |
| 15-acetyl-DON | 17055 | 31369 | 1.84 | 269862 | 517570 | 1.92 |
| 3-acetyldeoxyvinalenol | 13353 | 22613 | 1.69 | 179204 | 296396 | 1.65 |
| Aflatoxin G2 | 171597 | 262824 | 1.53 | 3429501 | 5274354 | 1.54 |
| Aflatoxin G1 | 224058 | 304389 | 1.36 | 4607959 | 6102959 | 1.32 |
| ZON | 25617 | 37162 | 1.45 | 656915 | 927455 | 1.41 |
| Aflatoxin B2 | 159389 | 295648 | 1.85 | 3462489 | 5724754 | 1.65 |
| Aflatoxin B1 | 265935 | 223520 | 0.84 | 5335576 | 4425821 | 0.83 |
| Alpha-zearalenol | 16202 | 30224 | 1.87 | 382092 | 702420 | 1.84 |
| Deoxynivalenol | 6935 | 17346 | 2.50 | 117927 | 281906 | 2.39 |
| Nivalenol | 1790 | 4182 | 2.34 | 25276 | 64495 | 2.55 |
| Altenuene | 63224 | 113850 | 1.80 | 1187958 | 2059700 | 1.73 |
| Alternariol monomethyl ether | 19537 | 31024 | 1.59 | 428922 | 640689 | 1.49 |
| Alternariol | 48204 | 73272 | 1.52 | 837410 | 1302192 | 1.56 |
| Citrinin | 499900 | 1007880 | 2.02 | 5031182 | 9828890 | 1.95 |
| Tenuazonic acid | 21503 | 47828 | 2.22 | 89293 | 197658 | 2.21 |
While our pesticides and mycotoxins experiments showed that columns made with inert hardware can improve chromatographic performance, the independent results from Carrera and Wuppermann are even more compelling. Based on the experiences of these well-regarded scientists, in addition to our own testing, it seems clear that Restek’s Inert LC columns have many potential benefits to laboratories analyzing pesticides and mycotoxins. It will be interesting to see what other analyses may be improved as well!