Key Highlights
- Next-generation TriMax deactivation creates a robust and exceptionally neutral sample flow path.
- Maximum inertness improves peak shape for a wide range of semivolatiles, allowing lower calibration ranges and picogram-level sensitivity.
- Maximum column performance supports expanded analyte lists and scaled down sample extraction volumes.

Abstract
This study explores the impact of groundbreaking TriMax deactivation on the GC-MS/MS analysis of semivolatiles by EPA Method 8270E. The performance of four commonly used analytical columns was compared using peak asymmetry, calibration linearity, recovery, and repeatability tests across a wide range of compound chemistries. Results show that RMX-5Sil MS columns, built with a new TriMax deactivation technology, have a significantly more inert surface that meets performance criteria across acidic, basic, and neutral semivolatiles whereas other columns met criteria only for certain classes of compounds. Competitive performance across a wide range of semivolatiles at low levels allows labs to streamline operations through microextraction sample preparation (e.g., EPA Method 3511) or method consolidation. The TriMax deactivation technology used on RMX-5Sil MS columns has overcome activity issues that are demonstrated to affect overall quantitative performance of competitor columns.
Introduction
Semivolatile organic compounds (SVOCs) are monitored in the environment globally due to their prevalence and the risks they pose to human health. GC-MS is a common approach for analyzing semivolatiles when following methods, such as U.S. EPA Method 8270, which covers the analysis of extracts from many types of environmental matrices, including solid waste, soil, air sampling media, and water samples. The latest revision, EPA Method 8270E [1], allows either GC-MS or GC-MS/MS to be used, and with the higher sensitivity of MS/MS detection, labs can meet lower detection limits and realize solvent and time savings by scaling down sample extraction volumes via microextractions. Microextraction methods, such as EPA Method 3511 [2], are generally simpler and use less solvent (e.g., dichloromethane) than standard extraction methods. However, the extracts are less concentrated, which is driving the adoption of GC-MS/MS in laboratories seeking to reduce solvent consumption while maintaining method performance capabilities.
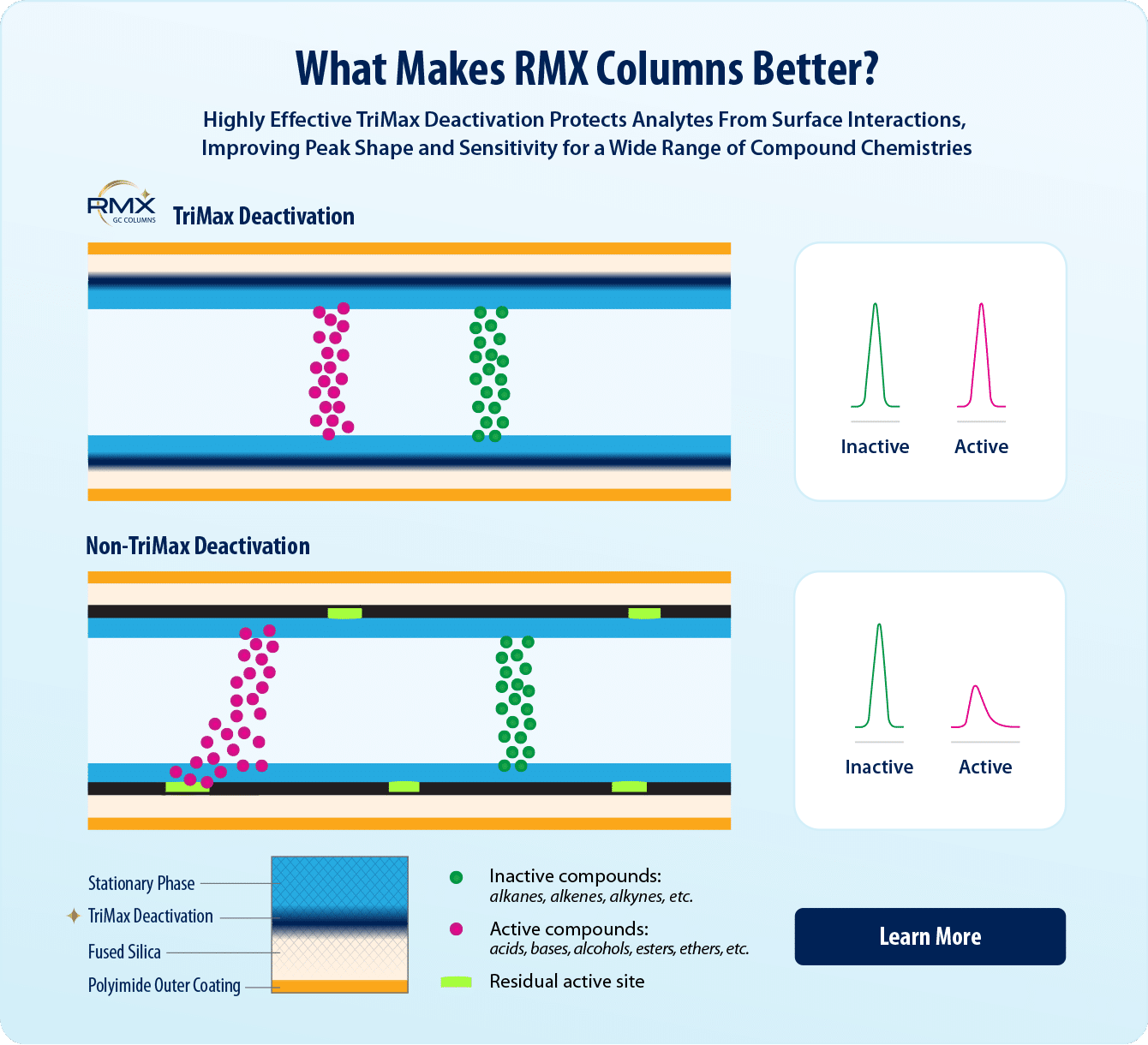
Semivolatiles comprise a wide range of compound chemistries, some of which are known to interact with active sites (e.g., silanols) in the sample flow path, which makes reliable low-level detection difficult. To overcome these challenging interactions, labs may use different GC columns for different target analytes. Midpolarity “5” or “5sil” type columns are commonly used, but as labs seek to lower detection limits and push the sensitivity of their columns, the drawbacks of the different manufacturing processes for each “5-type” column become more apparent. While neutral compounds are not typically affected by the active sites left behind on the GC column surface, acidic and basic compounds can be sensitive to even the most subtle differences in surfaces. With basic (benzidine) and acidic (pentachlorophenol) compounds included in system suitability checks, in addition to demand for increasingly sensitive calibrations, active sites on the GC column surface are coming under intense scrutiny. GC columns with a broadly effective deactivation are highly desired because by producing better results for acidic, basic, and neutral semivolatiles, they can allow labs to improve productivity and profitability through lower solvent consumption, better instrument performance, and more opportunity to consolidate methods.
In this study, we compared the performance of several GC columns that are widely used for semivolatiles analysis to the performance of RMX-5Sil MS columns. RMX-5Sil MS columns undergo a unique column surface deactivation featuring TriMax technology that produces a robust polymer deposition, eliminating active sites and resulting in an exceptionally inert sample flow path. The RMX-5Sil MS column uses a traditional 5sil polymer, so it is a direct 5sil replacement, while the highly neutral surface improves peak shape and performance for a wide variety of compounds to help lower calibration curves while still meeting data quality requirements.
Experimental
Standard and Sample Preparation
Calibration standards containing acidic, basic, and neutral semivolatiles were prepared in dichloromethane across a range of 0.5-5000 ppb to determine the lowest linear calibration range for each analyte. Fresh calibration standards were prepared for each column. Mid-range recovery test standards were also prepared at 50 ppb.
Instrument Conditions
Samples were run on four types of columns (RMX-5Sil MS, two premium competitor columns, and one traditional competitor column) in 30 m, 0.25 mm ID, 0.25 μm formats. A Thermo TRACE 1310 GC paired with a TSQ 8000 mass spectrometer was used for semivolatiles analysis under the conditions listed below. The conditions used are simplified to allow for standardization and direct comparison of the columns that were tested. Routine work may use different inlet, oven, or detector settings to further improve chromatographic results. Optimized conditions are demonstrated in a separate low-level study that included an expanded list of 150 commonly analyzed semivolatiles [3].
Injection volume: 1 µL
Liner: Topaz 4 mm Precision inlet liner with wool (cat.# 23267)
Injection port: 250 °C; 5:1 split; 1.2 mL/min
Carrier gas: helium
Oven: 40 °C (hold 1 min) to 280 °C at 12.4 °C/min to 315 °C at 3.3 °C/min (hold 1 min)
Detector: MS/MS; SRM mode; 280 °C transfer line temp; 330 °C source (see Figure 1 for SRM transitions)
Data Quality Evaluation
Data quality was assessed based on asymmetry, linearity, recovery, and repeatability, and general performance for each metric was categorized according to the criteria in Table I. Asymmetry was evaluated for each compound as a measurement of inertness. Linearity was assessed using both R2 and %RSD. Percent recovery was determined for each analyte at the lowest calibration point (LCP) and at 50 ppb, which was used as a midpoint recovery test. Repeatability of recovery was also determined at 50 ppb. Note that while linearity %RSD, R2, LCP recovery, and 50 ppb recovery were evaluated based on compliance with EPA Method 8270E criteria, recovery %RSD does not have a method-based compliance metric and asymmetry does not for most compounds, so data quality criteria were based on similar metrics in the method.
Table I: Data Quality Classifications
| Ideal | Acceptable | Poor | |
|---|---|---|---|
| Asymmetry | 0.9-1.2 | 0.5-0.9 or 1.2-2 | <0.5, >2 |
| Linearity (R2) | >0.995 | 0.990-0.995 | <0.990 |
| Linearity (%RSD) | <10% | 11-20% | >20% |
| Recovery (LCP) | 70-130% | 50-69% or 131-200% | <50%, >200% |
| Recovery (50 ppb) | 70-130% | 50-69% or 131-200% | <50%, >200% |
| Repeatability (%RSD at 50 ppb) | <10% | 11-20% | >20% |
Results and Discussion
Chromatographic Performance
Silanols are a common source of active sites on the fused silica surface of GC columns, so column manufacturers use different deactivation treatments to reduce their impact. However, if the deactivation is not thoroughly effective, an analyte’s interaction with silanols can cause inconsistent or delayed partitioning from the stationary phase, which results in peak tailing and subsequent issues, such as retention time shifts and poor sensitivity. Acidic compounds, particularly phenols, can be retained by silanols through hydrogen bonding, while basic compounds, such as benzidines, may bond to the surface through an acid-base interaction. One of the challenges with analyzing semivolatiles is that they include a wide range of compound classes that interact through different mechanisms and severities, so the column deactivation must be broadly effective.
To better combat silanol activity, Restek has developed next-generation TriMax deactivation for all RMX columns, which minimizes surface silanol activity though a strong polymer-fused silica interface, preventing analyte-silanol interactions. As shown in Figure 1, this results in excellent chromatographic performance and sharp, symmetric peaks for a wide range of semivolatiles, including well-known challenging compounds, such as 2,4-dinitrophenol (2,4-DNP); pentachlorophenol; and benzidine.
Figure 1: Excellent peak shape was obtained for a wide range of semivolatiles on an RMX-5Sil MS column under the standardized conditions used for comparative analysis. (For optimized conditions, see our low-level method for 150 semivolatiles [3].)
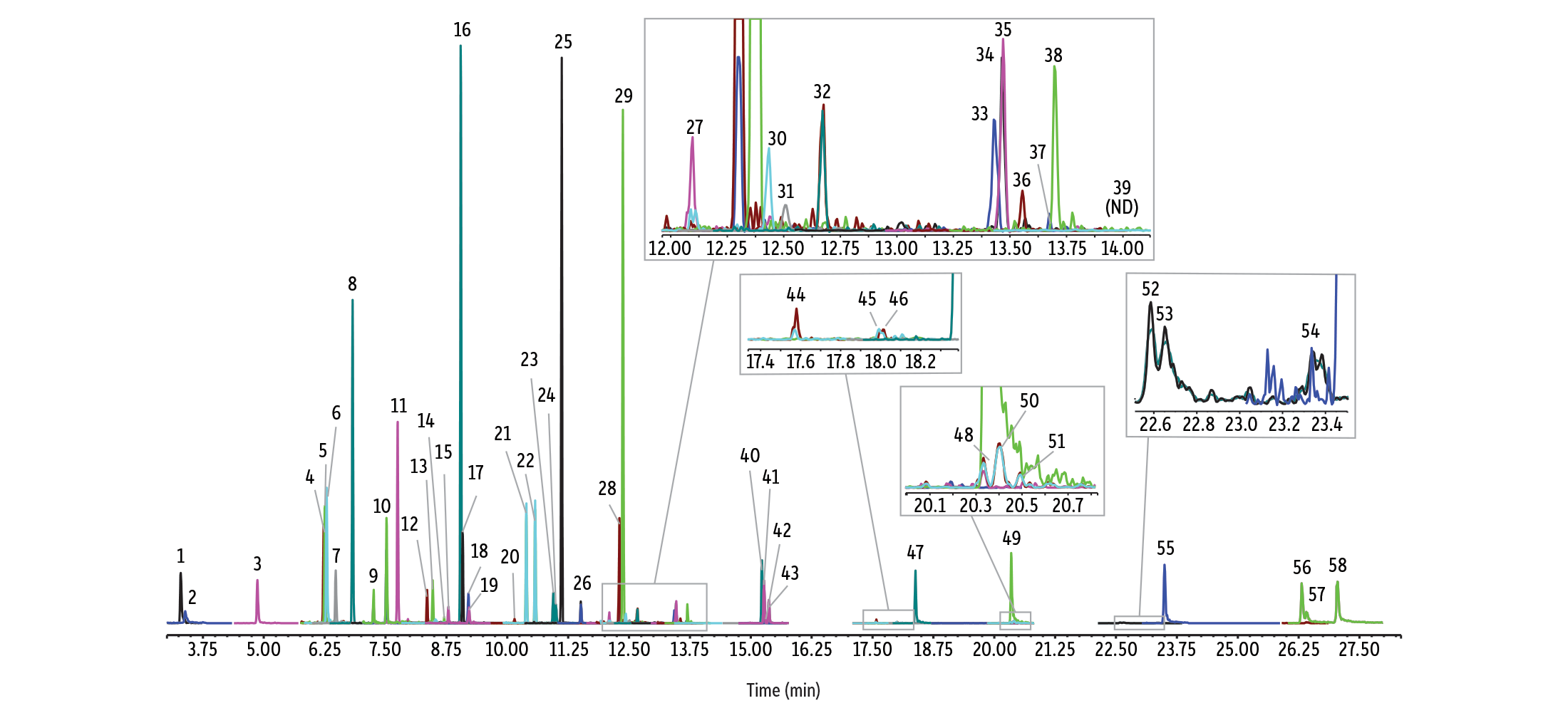
GC_EV1528
Peaks
| Peaks | tR (min) | Conc. (ng/mL) | Mass 1 | Product 1 | Collision energy 1 | Mass 2 | Product 2 | Collision energy 2 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. | N-Nitrosodimethylamine | 3.55 | 50 | 74 | 44 | 6 | 74 | 42 | 16 |
| 2. | Pyridine | 3.59 | 50 | 79 | 51 | 26 | 52 | 26 | 18 |
| 3. | 2-Fluorophenol | 5.03 | 50 | 112 | 92 | 6 | 92 | 63 | 14 |
| 4. | Phenol-d6 | 6.37 | 50 | 99 | 71 | 8 | 99 | 69 | 18 |
| 5. | Phenol | 6.40 | 50 | 94 | 66 | 10 | 94 | 65 | 16 |
| 6. | Aniline | 6.45 | 50 | 93 | 66 | 10 | 93 | 65 | 20 |
| 7. | 2-Chlorophenol | 6.62 | 50 | 128 | 64 | 14 | 128 | 63 | 24 |
| 8. | 1,4-Dichlorobenzene-d4 | 6.96 | 100 | 150 | 115 | 14 | 150 | 78 | 26 |
| 9. | 2-Methylphenol | 7.38 | 50 | 108 | 80 | 8 | 108 | 77 | 24 |
| 10. | 3- and 4-Methylphenol | 7.66 | 50 | 70 | 43 | 6 | 107 | 77 | 14 |
| 11. | Nitrobenzene-d5 | 7.89 | 50 | 128 | 82 | 12 | 128 | 54 | 28 |
| 12. | 2-Nitrophenol | 8.54 | 50 | 139 | 81 | 12 | 139 | 109 | 8 |
| 13. | 2,4-Dimethylphenol | 8.61 | 50 | 122 | 107 | 12 | 122 | 77 | 20 |
| 14. | Benzoic acid | 8.72 | 50 | 122 | 105 | 8 | 122 | 77 | 20 |
| 15. | 2,4-Dichlorophenol | 8.95 | 50 | 162 | 63 | 24 | 162 | 98 | 12 |
| 16. | Naphthalene-d8 | 9.18 | 100 | 136 | 134 | 14 | 136 | 108 | 18 |
| 17. | Naphthalene | 9.23 | 50 | 128 | 102 | 16 | 129 | 103 | 14 |
| 18. | 4-Chloroaniline | 9.35 | 50 | 127 | 100 | 10 | 127 | 65 | 20 |
| 19. | 2,6-Dichlorophenol | 9.37 | 50 | 162 | 63 | 24 | 164 | 63 | 26 |
| 20. | 4-Chloro-3-methylphenol | 10.28 | 50 | 142 | 107 | 12 | 107 | 77 | 12 |
| 21. | 2-Methylnaphthalene | 10.53 | 50 | 141 | 115 | 16 | 141 | 89 | 30 |
| 22. | 1-Methylnaphthalene | 10.71 | 50 | 141 | 115 | 16 | 141 | 89 | 30 |
| 23. | 2,4,6-Trichlorophenol | 11.09 | 50 | 132 | 97 | 10 | 196 | 97 | 24 |
| 24. | 2,4,5-Trichlorophenol | 11.09 | 50 | 132 | 97 | 10 | 196 | 97 | 24 |
| 25. | 2-Fluorobiphenyl | 11.26 | 50 | 172 | 171 | 12 | 172 | 170 | 22 |
| 26. | o-Nitroaniline | 11.66 | 50 | 138 | 92 | 12 | 138 | 65 | 22 |
| 27. | Acenaphthylene | 12.24 | 50 | 152 | 102 | 26 | 152 | 76 | 36 |
| 28. | 3-Nitroaniline | 12.49 | 50 | 138 | 92 | 12 | 138 | 65 | 20 |
| 29. | Acenaphthene-d10 | 12.53 | 100 | 162 | 160 | 18 | 164 | 162 | 14 |
| 30. | Acenaphthene | 12.60 | 50 | 153 | 126 | 36 | 153 | 77 | 38 |
| 31. | 2,4-Dinitrophenol | 12.70 | 50 | 184 | 154 | 6 | 154 | 79 | 12 |
| 32. | 4-Nitrophenol | 12.90 | 50 | 139 | 109 | 6 | 139 | 81 | 14 |
| 33. | 2,3,4,6-Tetrachlorophenol | 13.21 | 50 | 232 | 168 | 12 | 234 | 131 | 24 |
| 34. | 4-Nitroaniline | 13.60 | 50 | 138 | 108 | 8 | 138 | 80 | 18 |
| 35. | Fluorene | 13.61 | 50 | 165 | 115 | 24 | 165 | 139 | 26 |
| 36. | 4,6-Dinitro-2-methylphenol | 13.71 | 50 | 198 | 168 | 6 | 198 | 121 | 10 |
| 37. | Diphenylamine | 13.88 | 50 | 169 | 66 | 22 | 170 | 66 | 22 |
| 38. | 2,4,6-Tribromophenol | 14.10 | 50 | 330 | 141 | 36 | 332 | 143 | 34 |
| 39. | Pentachlorophenol | 15.04 | 50 | 228 | 165 | 14 | 270 | 169 | 22 |
| 40. | Phenanthrene-d10 | 15.40 | 100 | 188 | 160 | 20 | 184 | 156 | 22 |
| 41. | Phenanthrene | 15.46 | 50 | 178 | 152 | 18 | 178 | 151 | 32 |
| 42. | Dinoseb | 15.49 | 50 | 163 | 116 | 14 | 240 | 211 | 8 |
| 43. | Anthracene | 15.54 | 50 | 178 | 152 | 18 | 177 | 151 | 18 |
| 44. | Fluoranthene | 17.78 | 50 | 202 | 176 | 26 | 202 | 152 | 30 |
| 45. | Benzidine | 18.05 | 50 | 184 | 156 | 18 | 184 | 166 | 16 |
| 46. | Pyrene | 18.20 | 50 | 200 | 174 | 22 | 200 | 149 | 34 |
| 47. | p-Terphenyl-d14 | 18.57 | 50 | 244 | 242 | 14 | 244 | 240 | 22 |
| 48. | 3,3′-Dichlorobenzidine | 20.53 | 50 | 252 | 154 | 26 | 252 | 181 | 22 |
| 49. | Chrysene-d12 | 20.55 | 100 | 240 | 238 | 14 | 240 | 236 | 30 |
| 50. | Benz[a]anthracene | 20.63 | 50 | 228 | 202 | 22 | 226 | 200 | 28 |
| 51. | Chrysene | 20.63 | 50 | 228 | 202 | 22 | 228 | 201 | 36 |
| 52. | Benzo[b]fluoranthene | 22.90 | 50 | 252 | 226 | 22 | 250 | 224 | 24 |
| 53. | Benzo[k]fluoranthene | 22.91 | 50 | 252 | 226 | 22 | 250 | 224 | 24 |
| 54. | Benzo[a]pyrene | 23.66 | 50 | 252 | 226 | 22 | 250 | 224 | 26 |
| 55. | Perylene-d12 | 23.81 | 100 | 264 | 262 | 20 | 264 | 260 | 34 |
| 56. | Indeno[1,2,3-cd]pyrene | 26.74 | 50 | 276 | 274 | 38 | 276 | 250 | 30 |
| 57. | Dibenz[a,h]anthracene | 26.80 | 50 | 139 | 126 | 8 | 139 | 113 | 14 |
| 58. | Benzo[ghi]perylene | 27.47 | 50 | 276 | 274 | 38 | 138 | 125 | 12 |
Conditions
| Column | RMX-5Sil MS, 30 m, 0.25 mm ID, 0.25 µm (cat.# 17323) |
|---|---|
| Standard/Sample | |
| 8270 Calibration mix #1 (cat.# 31618) | |
| 8270 Calibration mix #2 (cat.# 31619) | |
| 8270 Calibration mix #5 (cat.# 31995) | |
| Base neutral surrogate mix (4/89 SOW) (cat.# 31024) | |
| Acid surrogate mix (4/89 SOW) (cat.# 31025) | |
| Revised SV internal standard mix (cat.# 31886) | |
| Diluent: | Dichloromethane |
| Conc.: | 50 ppb (100 ppb internal standards) |
| Injection | |
| Inj. Vol.: | 1 µL split (split ratio 5:1) |
| Liner: | Topaz 4.0 mm ID Precision liner w/wool (cat.# 23267) |
| Inj. Temp.: | 250 °C |
| Split Vent Flow Rate: | 6 mL/min |
| Oven | |
| Oven Temp.: | 40 °C (hold 1 min) to 280 °C at 12.4 °C/min to 315 °C at 3.3 °C/min (hold 1 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 1.2 mL/min @ 40 °C |
| Detector | SRM/MRM |
|---|---|
| Acquisition Type: | SRM/MRM |
| Source Temp.: | 330 °C |
| Transfer Line Temp.: | 280 °C |
| Analyzer Type: | Triple Quadrupole |
| Ionization Mode: | EI |
| Collision Gas: | Ar |
| Tune Type: | PFTBA |
| Tune Emission Current: | 70 μA |
| Instrument | Thermo Scientific TSQ 8000 Triple Quadrupole GC-MS |
| Sample Preparation | Standards were combined and diluted to a concentration of 50 ppb with internal standards added at 100 ppb. |
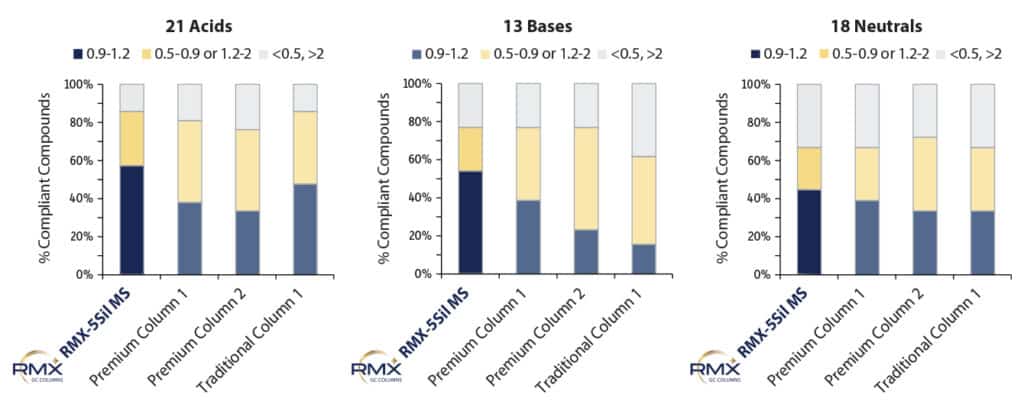
To compare the inertness of RMX-5Sil MS columns to other columns commonly used for semivolatiles analysis, we assessed peak asymmetry under standardized conditions at 50 ppb. The 50 ppb level was chosen for comparison so asymmetry could be evaluated at a midpoint calibration level. Evaluation at a midpoint compensates for tailing peak shapes that may blend into the baseline at low levels. Figure 2 demonstrates that for acidic, basic, and neutral semivolatiles, ideal results were obtained for more compounds on the RMX-5Sil MS column than on any of the other columns tested. The difference was especially pronounced for acidic and basic compounds, allowing more accurate identification and quantification for a wider range of semivolatiles in single run, which provides labs with opportunities for method consolidation.
Figure 2: An evaluation of asymmetry shows that exceptionally inert RMX-5Sil MS columns produce ideal peak shape results for more acidic, basic, and neutral semivolatiles than other analytical columns.
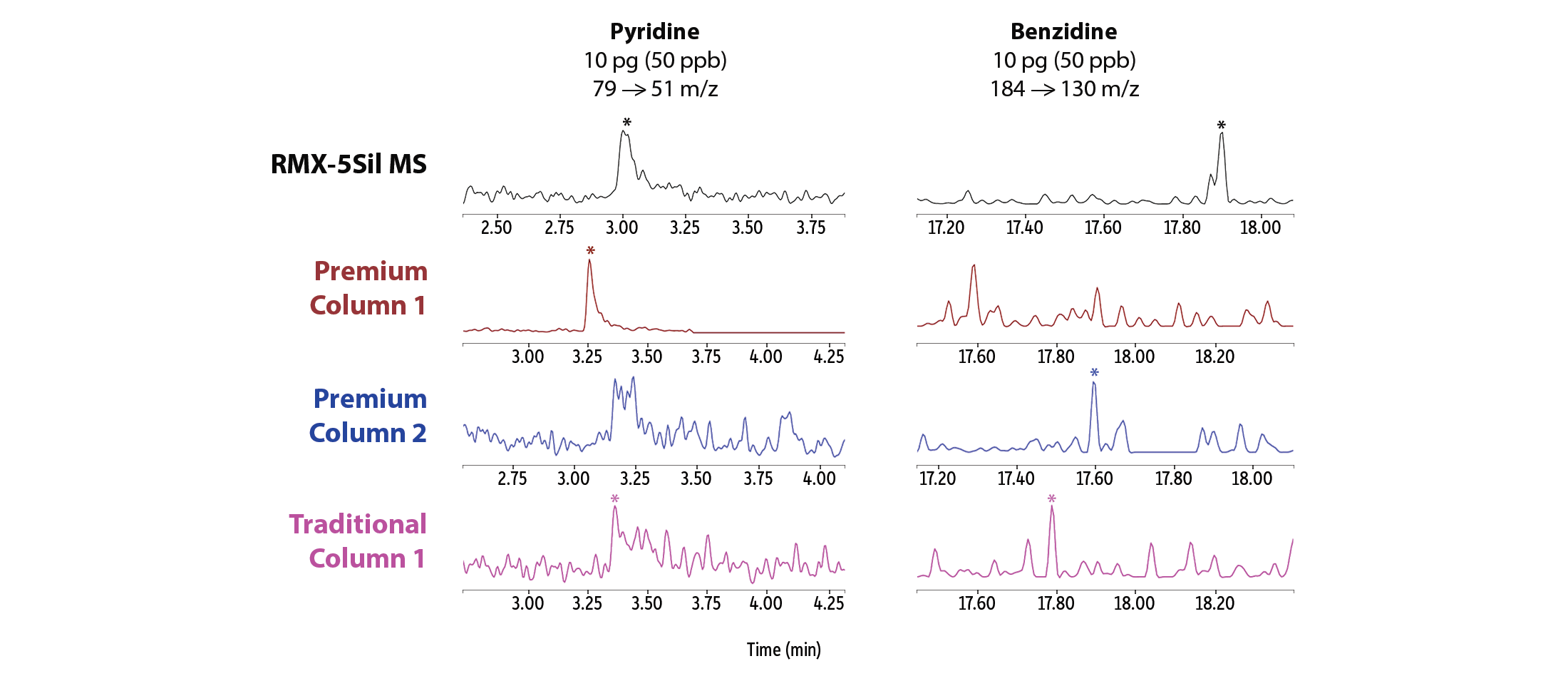
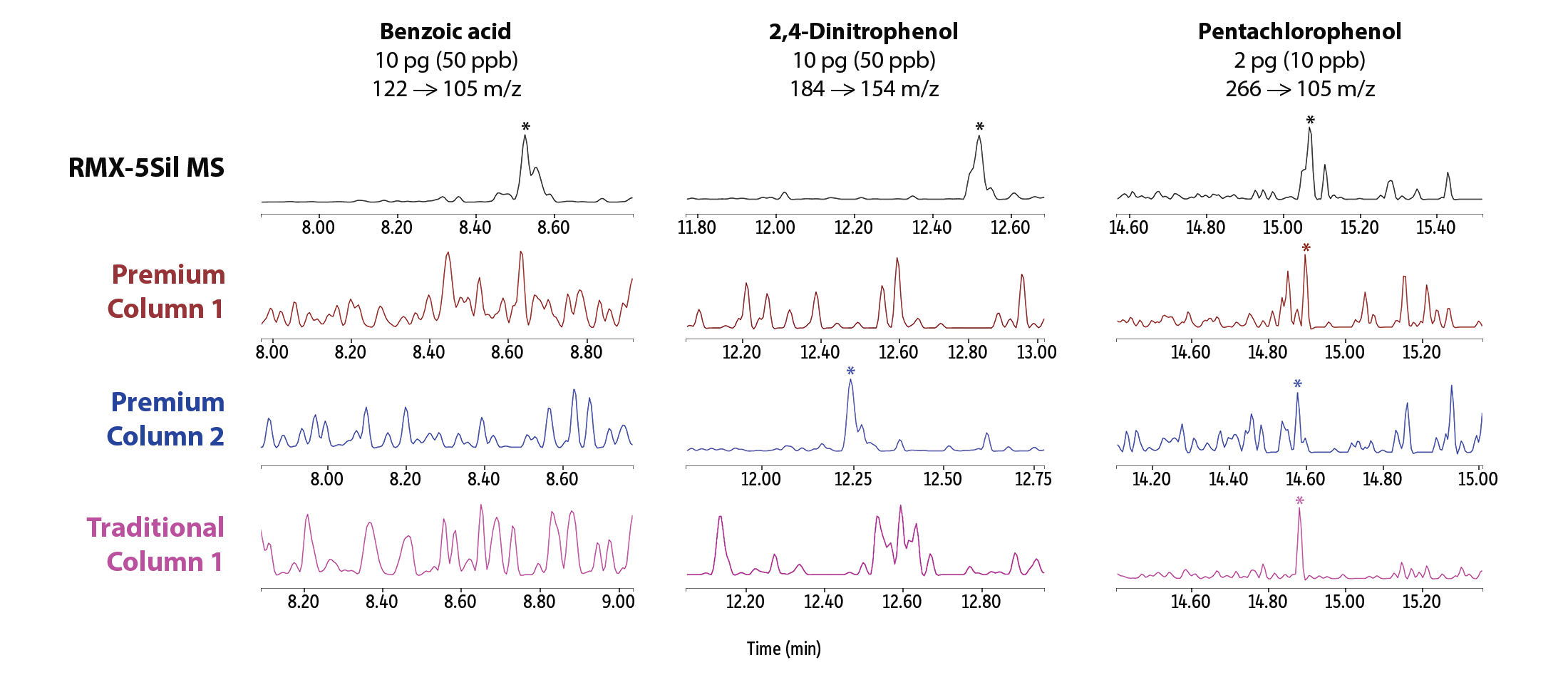
In addition to an overall improvement in peak asymmetry, better peak signals were seen at very low levels across a range of semivolatiles that are known to be particularly problematic (Figure 3). Good signals that allow for easy software integration were obtained for pyridine, benzidine, benzoic acid, 2,4-dinitrophenol, and pentachlorophenol on the RMX-5Sil MS column, whereas competitor columns showed similar performance for some compounds but notably compromised performance for others. Visual disparities among peak shapes illustrate how the inertness of the RMX TriMax deactivation is more broadly effective than the traditional deactivations used in other columns.
Figure 3: Broadly inert RMX-5Sil MS columns consistently produced good peak signals at the lowest calibration point (pg on-column) for a wide range of problematic active analytes.
Basic Compounds
Acidic Compounds

Linearity
Peak symmetry improves integration and increases the subsequent signal-to-noise ratio, which can allow for linear calibration at lower levels. The linear calibration ranges for each semivolatile were established on the RMX-5Sil MS column and varied by analyte (Table II). The number of calibration points ranged 5-11, and the lowest calibration level ranged 0.5-100 ppb (0.1-20 pg on column). Calibration linearity is sensitive to peak integration, so integration settings were kept consistent for each column. As peaks get closer to the baseline or tail excessively, manual integration becomes necessary, resulting in more analyst time per sample. Additional calibration models can be applied, such as weighted linear curves or quadratic curves, but an ideal calibration would be linear. By using a linear model for each analyte, the role of peak integration becomes more important. However, it should be noted that the lowest linear calibration point is not necessarily representative of the lowest detection limit.
Table II: Calibration Range and Number of Calibration Points for Each Semivolatile Compound
| Name | Min (ppb) | Max (ppb) | #Points |
|---|---|---|---|
| N-Nitrosodimethylamine | 2 | 1000 | 8 |
| Pyridine | 50 | 2000 | 6 |
| 2-Fluorophenol | 0.5 | 20 | 6 |
| Phenol-d6 | 2 | 200 | 6 |
| Phenol | 2 | 200 | 5 |
| Aniline | 2 | 200 | 6 |
| 2-Chlorophenol | 0.5 | 200 | 7 |
| 2-Methylphenol | 5 | 200 | 6 |
| 3- and 4-Methylphenol | 2 | 1000 | 8 |
| Nitrobenzene-d5 | 5 | 1000 | 6 |
| 2-Nitrophenol | 5 | 200 | 5 |
| 2,4-Dimethylphenol | 2 | 200 | 6 |
| Benzoic acid | 100 | 2000 | 5 |
| 2,4-Dichlorophenol | 2 | 2000 | 9 |
| Naphthalene | 1 | 50 | 5 |
| 2,6-Dichlorophenol | 1 | 100 | 5 |
| 4-Chloroaniline | 2 | 200 | 6 |
| 4-Chloro-3-methylphenol | 5 | 2000 | 8 |
| 2-Methylnaphthalene | 1 | 2000 | 10 |
| 1-Methylnaphthalene | 1 | 2000 | 10 |
| 2,4,6-Trichlorophenol | 5 | 1000 | 7 |
| 2,4,5-Trichlorophenol | 10 | 2000 | 8 |
| 2-Fluorobiphenyl | 0.5 | 2000 | 11 |
| o-Nitroaniline | 1 | 100 | 6 |
| Acenaphthylene | 5 | 200 | 5 |
| 3-Nitroaniline | 2 | 100 | 6 |
| Acenaphthene | 5 | 2000 | 7 |
| 2,4-Dinitrophenol | 50 | 2000 | 6 |
| 4-Nitrophenol | 5 | 2000 | 8 |
| 2,3,4,6-Tetrachlorophenol | 10 | 2000 | 7 |
| Fluorene | 5 | 2000 | 9 |
| 4-Nitroaniline | 2 | 200 | 6 |
| 4,6-Dinitro-2-methylphenol | 5 | 100 | 5 |
| Diphenylamine | 5 | 200 | 5 |
| 2,4,6-Tribromophenol | 20 | 2000 | 7 |
| Pentachlorophenol | 10 | 1000 | 6 |
| Phenanthrene | 1 | 200 | 7 |
| Dinoseb | 10 | 200 | 5 |
| Anthracene | 5 | 200 | 6 |
| Fluoranthene | 10 | 200 | 5 |
| Benzidine | 50 | 2000 | 5 |
| Pyrene | 5 | 100 | 5 |
| p-Terphenyl-d14 | 1 | 200 | 7 |
| 3,3′-Dichlorobenzidine | 20 | 200 | 5 |
| Benz[a]anthracene | 20 | 500 | 5 |
| Chrysene | 10 | 1000 | 5 |
| Benzo[b]fluoranthene | 20 | 1000 | 5 |
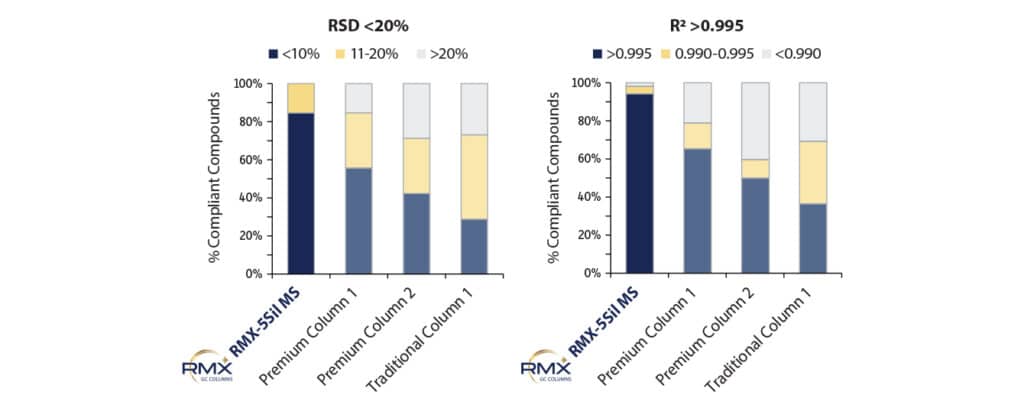
After the linear calibration curves were established on the RMX-5Sil MS column, fresh calibration curves were run on each column using the same calibration range and integration settings. Linear fit for each calibration curve was compared using R2 (unweighted) and response factor %RSD per EPA Method 8270E criteria. Results showed that the RMX-5Sil MS column produced method-compliant results for more compounds than the other columns did (Figure 4). Superior R2 and %RSD values on the RMX-5Sil MS column demonstrate better overall calibration curve linearity, which will improve quantitative precision and accuracy.
Figure 4: More semivolatile compounds meet data quality objectives (>0.995 R2 and <10% RSD) on RMX-5Sil MS columns than on other columns, demonstrating superior calibration linearity.
Recovery
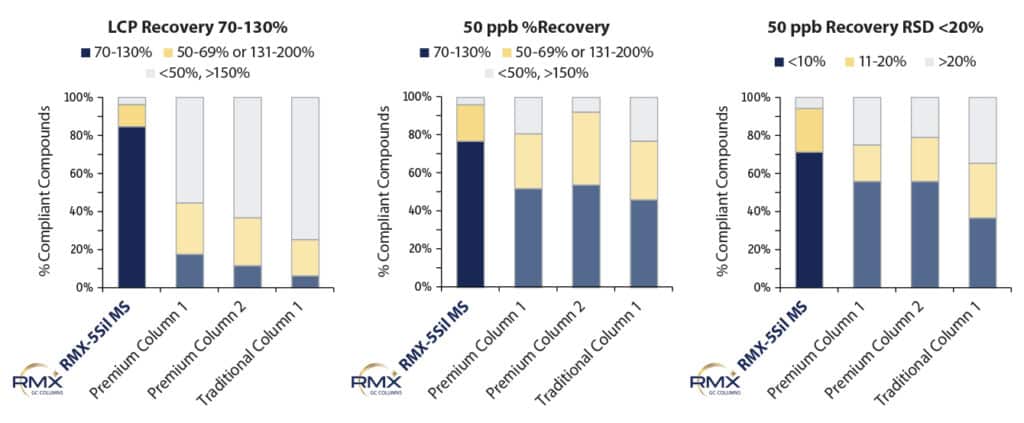
Recovery was determined at both the lowest calibration point and at the 50 ppb midpoint level to assess the impact of column choice. Repeatability of recovery results was evaluated at the 50 ppb midpoint because it is more representative of column performance than the LCP, where peak shape is more likely to be influenced by baseline noise from the instrument. As shown in Figure 5, recoveries at the LCP were substantially better on the RMX-5Sil MS column than on the other columns tested, which can be attributed to a more effective deactivation that improves peak response and integration at trace levels. While recoveries were fairly similar across all columns at the 50 ppb midpoint, recovery results were much more consistent on the RMX-5Sil MS column. Repeatability is dependent on the relationship between peak shape and the integration algorithm, and small differences in peak shape can affect integration. The highly consistent results obtained on the RMX-5Sil MS column demonstrate that its TriMax deactivation minimizes surface activity that contributes to variability across injections.
Figure 5: TheRMX-5Sil MS column has the most compounds achieving 70-130% recovery at both the lowest calibration point (LCP) and at 50 ppb. In addition, it was highly consistent and has the most compounds achieving <10% RSD at 50 ppb.
Conclusion
While 5-type and 5sil-type columns have appropriate selectivity for semivolatiles analysis and are widely used around the world, traditional deactivations are not completely effective at blocking active sites. As a result, labs struggle to analyze active compounds, particularly at trace levels. Productivity may further suffer if multiple columns are needed for different analyte groups or when quality criteria begin to fail. Based on the comparative study presented here, the ground-breaking TriMax used in RMX-5Sil MS columns resulted in a significantly more inert column surface that gave superior performance across compound classes. As a result, when using RMX-5Sil MS columns, data quality objectives were met for more semivolatiles compared to other analytical columns. This improved performance for a wide range of acidic, basic, and neutral compounds gives labs more opportunity to reap the benefits of reduced solvent consumption and method consolidation relative to traditional 5-type columns.
References
- U.S. Environmental Protection Agency, Method 8270E, Semivolatile organic compounds by gas chromatography/mass spectrometry, June 2018. https://www.epa.gov/sites/default/files/2020-10/documents/method_8270e_update_vi_06-2018_0.pdf
- U.S. Environmental Protection Agency, Method 3511, Organic compounds in water by microextraction, July 2014. https://www.epa.gov/sites/default/files/2015-12/documents/3511.pdf
- E. Pack, C. English, R. Dhandapani, and C. Myers, Increase lab efficiency with a consolidated trace-level semivolatiles method, Featured application note, EVFA5253, Restek Corporation, 2025.