We frequently get a lot of questions related to the storage of cannabinoid standards. How long does an ampule stay “good” once opened? Can I store a mixed solution of acids and neutrals? Do I need to take precautions to protect my standards from light? These are all great questions as the general theme is a concern over data quality. After all, the quality of reported results can only be as good as the quality of the reference standards.
As for the answer to these questions, let’s start by first examining a scenario that we commonly encounter: storing cannabinoid calibration mixes in the autosampler. It’s easy and convenient, but what might happen using this approach?
To investigate this, three different mixes were made. The first mix was a standard containing nine neutral compounds including cannabichromene (CBC), cannabicyclol (CBL), cannabidiol (CBD), cannabidivarin (CBDV), cannabigerol (CBG), cannabinol (CBN), tetrahydrocannabinol (delta-8-THC), tetrahydrocannabinol (delta-9-THC) and tetrahydrocannabivarin (THCV). The second mix contained seven acidic cannabinoids including cannabidivarinic acid (CBDVA), cannabidiolic acid (CBDA), cannabigerolic acid (CBGA), tetrahydrocannabivarinic acid (THCVA), cannabinolic acid (CBNA), tetrahydrocannabinolic acid A (THCA-A), and cannabichromenic acid (CBCA). The final mix contained all sixteen acidic and neutral cannabinoids. Each cannabinoid was diluted to a final concentration of 50 ppm using water:ACN 25:75. A summary of each standard’s contents can be viewed in the table below.
| Neutral Compounds (9) | Acidic Compounds (7) | Combined Mix (16) |
|---|---|---|
| CBC | CBDVA | CBDVA |
| CBL | CBDA | CBDV |
| CBD | CBGA | CBDA |
| CBDV | THCVA | CBGA |
| CBG | CBNA | CBG |
| CBN | THCA-A | CBD |
| delta-8-THC | CBCA | THCV |
| delta-9-THC | THCVA | |
| THCV | CBN | |
| CBNA | ||
| delta-9-THC | ||
| delta-8-THC | ||
| CBL | ||
| CBC | ||
| THCA-A | ||
| CBCA |
Table 1. Summary of compounds in each standard made at 50 ppm concentration.
To begin, each mix was tested on the day it was made and stored in the autosampler at 10°C. After three days, each mix was re-tested. The testing continued for a total period of 37 days. The results were made into graphs by plotting the analyte area vs. number of days since the mix was first prepared. Trends were then observed to infer stability.
For the neutral mix, degradation was observed after 17 days of storage. The acidic mix was less robust and compounds started to degrade at day 7. The combination of neutral and acidic cannabinoids had similar results to the acidic mix by also showing signs of degradation at day 7.
An example of the graphs that were constructed to infer standard degradation can be seen in Figure 1. This graph represents the results collected over 37 days of testing for the acidic mix stored in the autosampler at 10⁰C. Day 7 to day 10 shows a change in the analyte distribution, where CBDVA goes from being at the highest area count to the second highest, a clear indicator of degradation. It should be noted that the autosampler vial cap was not initially replaced after Day 0 data was collected.
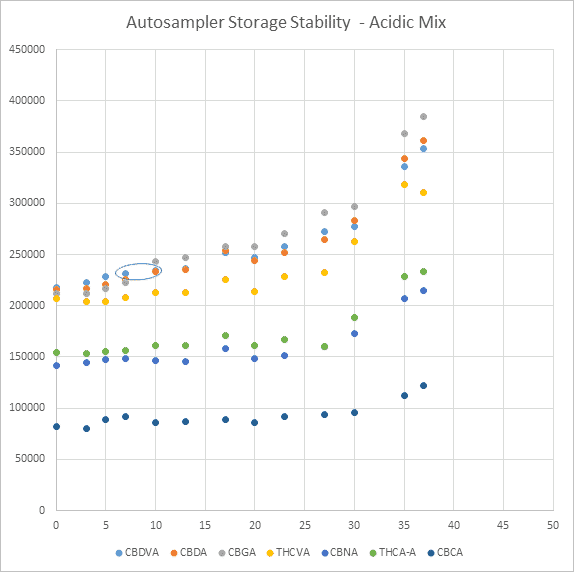
Figure 1. Graph of peak area vs. day since making standard plotted for acidic standard stored on autosampler at 10⁰C.
Keep in mind these results are for when standards are diluted in 25:75 water:ACN and do not represent the open-ampule stability of cannabinoids prepared in methanol or acetonitrile. This limited study sheds some light on the importance of replacing autosampler vial caps after injections have been made. As our autosampler was kept at 10°C, the impact of a being stored in an unchilled autosampler would only be more severe.
As for the questions posed in the introduction, we can provide more detailed answers in a future blog series, but our general recommendation is to keep acids and neutrals unmixed, stored at least at -20°C, protected from light, and capped with an intact septum. We highly recommend for everyone to perform stability studies for themselves in order to provide a record of the justification for assigned expiration dates of cannabinoid mixes.
How do you make your standards for potency testing? Are they made fresh before every use or are they stored?


