Many cannabinoids have emerged worldwide that labs are interested in integrating into their current potency methods. These include delta-9-THC isomers such as exo-THC, (6aR,9R)-delta-10-THC, and (6aR,9S)-delta-10-THC. Additional cannabinoids of interest include derivatives of delta-9-THC such as THCO-acetate, a synthetic psychoactive cannabinoid, THCP, the most potent cannabinoid in the cannabis plant to-date, and 9S-HHC/9R-HHC, which are the hydrogenated forms of delta-9-THC.
When analyzing cannabinoids by LC-UV/VIS the user is often limited by the chromatographic space available when adding cannabinoids to their methods, since all analytes must be resolved when using this detector. Restek offers many solutions for cannabinoid analysis using Raptor columns. Raptor, Restek’s superficially porous particle, is the gold-standard for highly efficient separations of cannabinoids. It is important to also discuss Ultra, one of Restek’s fully porous particles, for cannabinoid separations, and the differences compared to Raptor.
Fully porous particles have more surface area for the phase to be bonded and thus have a higher carbon load when compared to superficially porous particles. For comparison, the carbon load on a fully porous Ultra C18 3 μm particle is 20% compared to the carbon load on a Raptor ARC-18 2.7 μm which is 7%. Ultra C18 can also provide a cost-benefit compared to Raptor ARC-18 as it is priced more economically, but other factors such as analytical runtime, pressure, solvent consumption, etc. must also be considered. Reducing solvent consumption and instrument run time can be some of the benefits of using a Raptor ARC-18, and an example of this can be seen here for the analysis of 16 cannabinoids in 10 minutes.
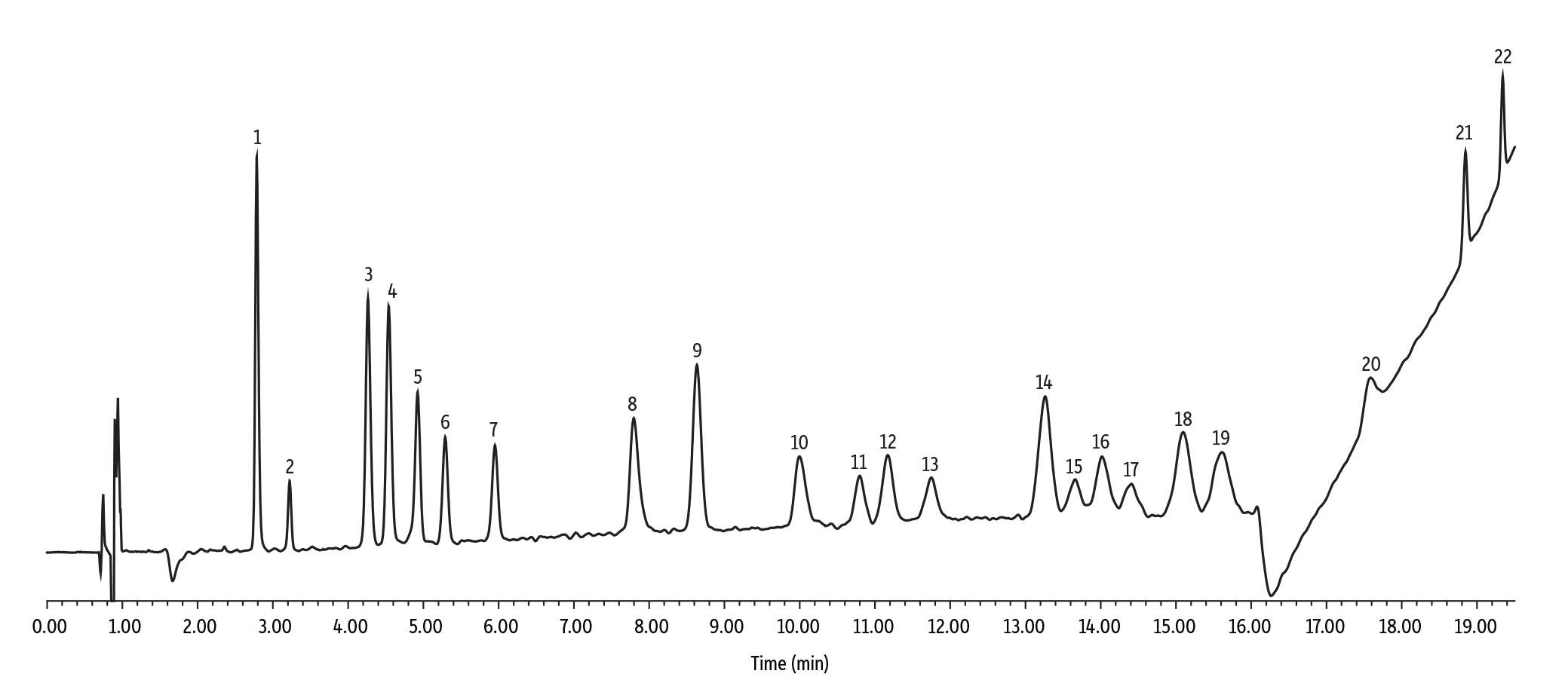
Now that we understand the differences of Raptor and Ultra C18 columns, let’s look at a very challenging cannabinoid separation. In this work, 22 commonly requested cannabinoids were analyzed by LC-VU/VIS with a cycle time of 21 minutes.
LC_FF0631
Peaks
| Peaks | tR (min) | |
|---|---|---|
| 1. | Cannabidivarinic acid (CBDVA) | 2.79 |
| 2. | Cannabidivarin (CBDV) | 3.22 |
| 3. | Cannabidiolic acid (CBDA) | 4.26 |
| 4. | Cannabigerolic acid (CBGA) | 4.54 |
| 5. | Cannabigerol (CBG) | 4.92 |
| 6. | Cannabidiol (CBD) | 5.29 |
| 7. | Tetrahydrocannabivarin (THCV) | 5.95 |
| 8. | Tetrahydrocannabivarinic acid (THCVA) | 7.80 |
| 9. | Cannabinol (CBN) | 8.64 |
| 10. | Cannabinolic acid (CBNA) | 10.00 |
| 11. | Exo-tetrahydrocannabinol (exo-THC) | 10.80 |
| Peaks | tR (min) | |
|---|---|---|
| 12. | d9-Tetrahydrocannabinol (9-THC) | 11.17 |
| 13. | d8-Tetrahydrocannabinol (8-THC) | 11.75 |
| 14. | (6aR,9S)-delta-10-Tetrahydrocannabinol (9S-10-THC) | 13.26 |
| 15. | 9(R)-Hexahydrocannabinol (9(R)-HHC) | 13.66 |
| 16. | (6aR,9R)-delta-10-Tetrahydrocannabinol (9R-10-THC) | 14.02 |
| 17. | 9(S)-Hexahydrocannabinol (9(S)-HHC) | 14.41 |
| 18. | Cannabichromene (CBC) | 15.09 |
| 19. | 9-Tetrahydrocannabinolic acid-A (THCA) | 15.62 |
| 20. | Cannabichromenic acid (CBCA) | 17.57 |
| 21. | 9-Tetrahydrocannabiphorol (THCP) | 18.85 |
| 22. | 9-Tetrahydrocannabinol Acetate (THCO-Acetate) | 19.34 |
Conditions
| Column | Ultra C18 (cat.# 9174365) | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 150 mm x 4.6 mm ID | ||||||||||||||||||||||||||||
| Particle Size: | 3 µm | ||||||||||||||||||||||||||||
| Pore Size: | 100 Å | ||||||||||||||||||||||||||||
| Guard Column: | UltraShield UHPLC precolumn filter, 0.2 µm frit (cat.# 25810) | ||||||||||||||||||||||||||||
| Temp.: | 30 °C | ||||||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||||||
| Cannabinoids acids 7 standard (cat.# 34144) | |||||||||||||||||||||||||||||
| Other standards were obtained separately. | |||||||||||||||||||||||||||||
| Diluent: | 25:75 Water:acetonitrile | ||||||||||||||||||||||||||||
| Conc.: | 50 ppm | ||||||||||||||||||||||||||||
| Inj. Vol.: | 5 µL | ||||||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||||||
| A: | Water:acetonitrile (25:75), 2.5 mM ammonium formate, 0.1% formic acid | ||||||||||||||||||||||||||||
| B: | Methanol, 0.1% formic acid | ||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
| Max Pressure: | 269 bar |
| Detector | UV/Vis @ 228 nm |
|---|---|
| Flow Cell Size: | 500 nL |
| Instrument | Waters ACQUITY UPLC H-Class |
| Sample Preparation | Standards were aliquoted into 2 mL, screw-thread vials (cat.# 21143) and capped with short-cap, screw-vial closures (cat.# 24498). |
By using this method, labs can achieve baseline separation for 22 cannabinoids. Is your lab interested in implementing this method? Contact your sales representative.



