Abstract
As interest grows in monitoring a wider range of PFAS in both potable and non-potable waters, efficient methodology becomes more important. Here, we developed a unique approach that provides concurrent ultrashort-chain PFAS analysis along with alternative and legacy PFAS, allowing C2, C3, C4, C6, C8, and alternative compounds to be tested together instead of through separate methods. Results from verification experiments are presented.
Introduction
Ultrashort-chain, or C2 and C3, per- and polyfluoroalkyl substances (PFAS) are small and very polar compounds that contribute to at least 40% of the total PFAS detected in environmental waters (e.g., rain, river, and groundwaters) [1, 2, 3]. Ultrashort-chain PFAS include trifluoroacetic acid (TFA), perfluoropropanoic acid (PFPrA), perfluoroethane sulfonate (PFEtS), and perfluoropropane sulfonate (PFPrS), with TFA being the most abundant and difficult to analyze chromatographically. Current practices for PFAS monitoring do not address the analysis of these newly trending ultrashort-chain compounds due to their insufficient retention on typical reversed-phase (RP) columns. On the other hand, analytical methods implementing anion-exchange chromatography often show too much retention and poor chromatographic performance for ultrashort-chain PFAS. The challenge becomes even greater for simultaneous monitoring of ultrashort-chain, alternative, and legacy PFAS in a single method.
To overcome this limitation, we used a unique hybrid HILIC/ion-exchange column (Raptor Polar X) to develop a fast and simple LC-MS/MS method for comprehensive analysis of C2, C3, C4, C6, C8, and alternative PFAS. Because the column employs balanced, multimode retention mechanisms, ultrashort-chain PFAS and long-chain PFAS can all be analyzed in a single isocratic run. This direct injection method was evaluated by precision and accuracy analysis of fortified water samples, including tap water, river water, groundwater, and water from publicly owned treatment works (POTW, sewage effluent). As demonstrated here, the method provides convenient setup and high-throughput conditions for water testing labs interested in adding ultrashort-chain PFAS analysis to the same workflow used to measure alternative and legacy PFAS.
Experimental
Chromatographic Method:
The chromatographic conditions were as follows. The transitions and internal standard used for each analyte are provided in Table I.
| Column: | Raptor Polar X (2.7 µm, 50 mm x 2.1 mm ID [cat.# 9311A52]) | |
| Column temp.: | 40 °C | |
| Injection volume: | 10 µL | |
| Mobile phase A: | Water, 10 mM ammonium formate, 0.05% formic acid | |
| Mobile phase B: | Acetonitrile:methanol (60:40), 0.05% formic acid | |
| Time (min) | %B | |
| 0.00 | 85 | |
| 8.00 | 85 | |
| Flow rate: | 0.5 mL/min | |
| Ion mode: | Negative ESI | |
| Mode: | MRM | |
Table I: Analyte MS Transitions for Ultrashort-Chain PFAS Analysis Concurrent with Alternative and Legacy PFAS in Water Samples.
| Analyte | Precursor Ion | Product Ion | IS for Quantification |
|---|---|---|---|
| TFA | 113.03 | 69.01 | 13C2-PFHxA |
| PFPrA | 163.03 | 119.01 | 13C2-PFHxA |
| PFBA | 212.97 | 168.97 | 13C2-PFHxA |
| PFHxA | 312.97 | 268.90 | 13C2-PFHxA |
| PFOA | 412.90 | 368.91 | 13C2-PFOA |
| HFPO-DA | 284.97 | 168.92 | 13C2-PFOA |
| ADONA | 376.90 | 250.93 | 13C2-PFOA |
| PFEtS | 198.98 | 79.92 | 13C3-PFBS |
| PFPrS | 248.97 | 79.98 | 13C3-PFBS |
| PFBS | 298.97 | 79.97 | 13C3-PFBS |
| PFHxS | 398.90 | 79.97 | 13C3-PFBS |
| PFOS | 498.84 | 79.97 | 13C4-PFOS |
| 9Cl-PF3ONS | 530.78 | 350.85 | 13C4-PFOS |
| 11Cl-PF3OUdS | 630.78 | 450.80 | 13C4-PFOS |
| 13C2-PFHxA | 314.97 | 269.93 | – |
| 13C2-PFOA | 414.90 | 369.87 | – |
| 13C3-PFBS | 301.90 | 79.97 | – |
| 13C4-PFOS | 502.84 | 79.97 | – |
Sample Preparation
In a polypropylene vial (used to mitigate background contamination), 250 µL of each water sample was mixed with 250 µL of methanol and 5 µL of internal standard solution (10 ng/mL of 13C2-PFHxA, 13C2-PFOA, 13C3-PFBS, 13C4-PFOS in methanol). The vial was capped with a polyethylene cap (again, to reduce background contamination) for injection and analysis.
Calibration standards were prepared by using deionized water (generated by a Thermo Scientific Barnstead E-Pure system) and fortifying it with 14 analytes at a range of 10–800 ng/L. The calibration standard solutions were then diluted 1:1 in methanol following the sample preparation procedure above.
A tap water sample from the Restek facility and three water samples (Chicago river water, groundwater, and POTW effluent water) supplied by the United States Environmental Protection Agency (U.S. EPA) were fortified at 40 and 160 ppt. Blank and fortified water samples were diluted 1:1 in methanol as above for chromatographic analysis and quantified with the calibration standards. For TFA measurement in groundwater, the sample was diluted fivefold with deionized water before fortification at 40 and 160 ppt due to its high TFA concentration.
Results & Discussion
Chromatographic Performance
An isocratic elution was established that produced a fast, simple ultrashort-chain PFAS analysis along with alternative and legacy PFAS in water samples. All analytes eluted in 4 minutes with balanced retention and good peak shapes (Figure 1). No matrix interference was observed in any of the water samples using an 8-minute cycling time. As will be discussed below, the approximately 4-minute hold after the last eluting compound was shown to be necessary to avoid possible matrix interferences.
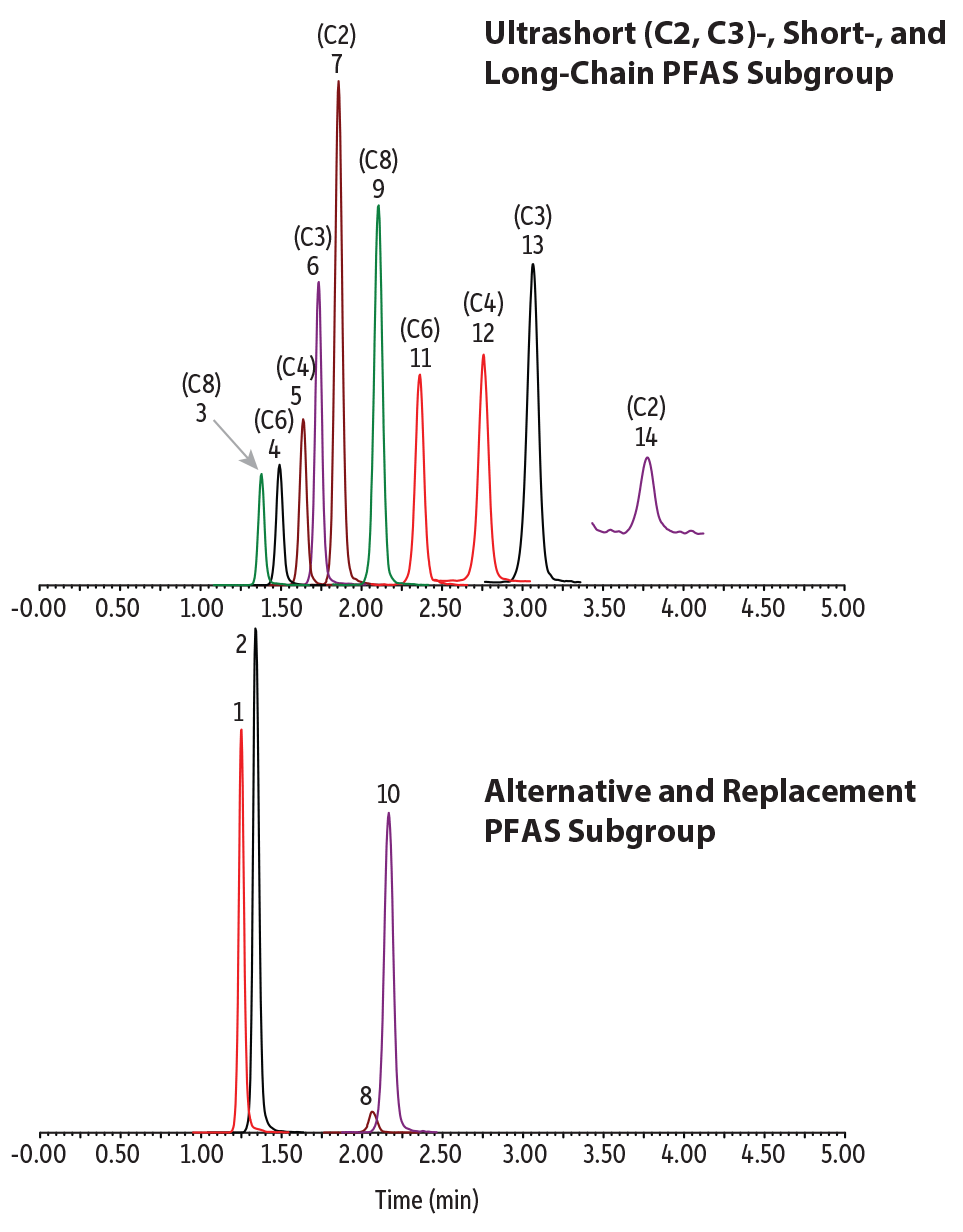
LC_EV0569
Peaks
| Peaks | tR (min) | Conc. (ng/L) | Precursor Ion | Product Ion | |
|---|---|---|---|---|---|
| 1. | 11-Chloroeicosafluoro-3-oxanonane-1-sulfonate (11CL-PF3OUdS) | 1.25 | 400 | 630.78 | 450.80 |
| 2. | 9-Chlorohexadecafluoro-3-oxanonane-1-sulfonate (9Cl-PF3ONS) | 1.34 | 400 | 530.78 | 350.85 |
| 3. | Perfluorooctanesulfonic acid (PFOS) | 1.38 | 400 | 498.84 | 79.97 |
| 4. | Perfluorohexanesulfonic acid (PFHxS) | 1.49 | 400 | 398.90 | 79.97 |
| 5. | Perfluorobutanesulfonic acid (PFBS) | 1.64 | 400 | 298.97 | 79.97 |
| 6. | Perfluoropropanesulfonic acid (PFPrS) | 1.73 | 400 | 248.97 | 79.98 |
| 7. | Perfluoroethanesulfonic acid (PFEtS) | 1.86 | 400 | 198.98 | 79.92 |
| Peaks | tR (min) | Conc. (ng/L) | Precursor Ion | Product Ion | |
|---|---|---|---|---|---|
| 8. | Hexafluoropropylene oxide dimer acid (HFPO-DA) | 2.06 | 400 | 284.97 | 168.92 |
| 9. | Perfluorooctanoic acid (PFOA) | 2.11 | 400 | 412.90 | 368.91 |
| 10. | Ammonium 4,8-dioxa-3H-perfluorononanoate (ADONA) | 2.15 | 400 | 376.90 | 250.93 |
| 11. | Perfluorohexanoic acid (PFHxA) | 2.36 | 400 | 312.97 | 268.90 |
| 12. | Perfluorobutanoic acid (PFBA) | 2.76 | 400 | 212.97 | 168.97 |
| 13. | Perfluoropropionic acid (PFPrA) | 3.06 | 400 | 163.03 | 119.01 |
| 14. | Trifluoroacetic acid (TFA) | 3.77 | 400 | 113.03 | 69.01 |
Linearity
The calibration range is 20–800 ppt for TFA and 10–800 ppt for all other analytes. Four internal standards were evaluated to determine the best fitting standard curve for the different analytes. All compounds showed acceptable linearity with r2 values >0.996, and deviations <20% using 1/x weighted quadratic regression.
Accuracy & Precision
In our initial experiments, matrix interference for the TFA signal was observed for water sample analysis performed using a 5-minute isocratic run. Different analysis times were tested, and it was determined that an 8-minute run time was needed to avoid matrix interference for all analytes. The isocratic hold time may need to be modified based on the specific instrumentation used and/or samples analyzed.
The blank water samples showed various levels of TFA, C3, C4, C6, and C8 PFAS with no detectable ADONA, HFPO-DA, 9Cl-PF3ONS, and 11Cl-PF3OUdS (Table II). An example chromatogram for ultrashort-chain PFAS analysis with concurrent determination of alternative and legacy PFAS in a blank POTW sample is shown in Figure 2.
Table II: Detectable analytes in blank water samples.
| Detected Concentration (ng/L) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | TFA | PFPrA | PFBA | PFHxA | PFOA | HFPO-DA | ADONA | PFEtS | PFPrS | PFBS | PFHxS | PFOS | 9Cl-PF3ONS | 11Cl-PF3OUdS |
| Tap Water | 164.2 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| River Water | 193.3 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Groundwater | 1425 | ND | ND | ND | 5.4 | ND | ND | ND | ND | 6.7 | 3.9 | ND | ND | ND |
| POTW Water | 352.8 | 9.6 | 15.3 | 93.5 | 20.4 | ND | ND | ND | ND | 6.8 | 6.7 | 9.6 | ND | ND |
ND: non-detectable
Figure 2: Detectable PFAS in blank POTW water.
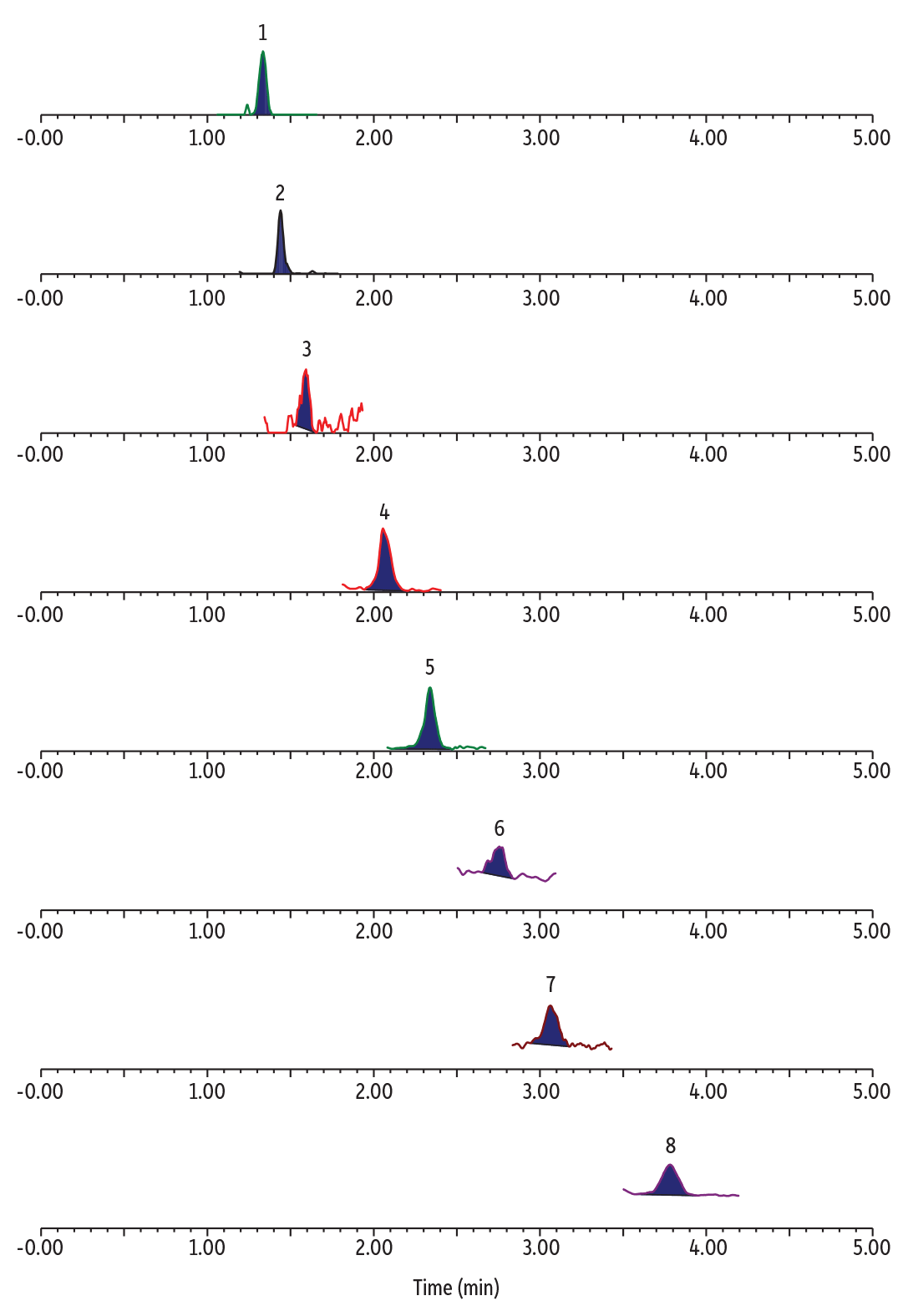
LC_EV0572
Peaks
| Peaks | tR (min) | Precursor Ion | Product Ion | |
|---|---|---|---|---|
| 1. | Perfluorooctanesulfonic acid (PFOS) | 1.35 | 498.84 | 79.97 |
| 2. | Perfluorohexanesulfonic acid (PFHxS) | 1.45 | 398.90 | 79.97 |
| 3. | Perfluorobutanesulfonic acid (PFBS) | 1.58 | 298.97 | 79.97 |
| 4. | Perfluorooctanoic acid (PFOA) | 2.05 | 412.90 | 368.91 |
| 5. | Perfluorohexanoic acid (PFHxA) | 2.34 | 312.97 | 268.90 |
| 6. | Perfluorobutanoic acid (PFBA) | 2.76 | 212.97 | 168.97 |
| 7. | Perfluoropropionic acid (PFPrA) | 3.06 | 163.03 | 119.01 |
| 8. | Trifluoroacetic acid (TFA) | 3.78 | 113.03 | 69.01 |
For accuracy determination (percent recovery), the measured amounts in the fortified samples were adjusted to account for the concentration in blank samples. Water samples were fortified at low and high concentration in duplicate for each analytical batch. A total of three analytical batches were measured on different days. Table III shows the accuracy and precision results calculated from the collection of all three batches of data. The method accuracy was demonstrated by recovery values being within 30% of the nominal concentration for both fortified and LLOQ levels in water samples. The %RSD was <20%, indicating acceptable method precision for ultrashort-chain PFAS analysis concurrent with alternative and legacy compounds in water.
Table III: Method Accuracy and Precision
| Average %Accuracy (%RSD) | |||||||||
| Matrices | Tap Water | River Water | Groundwater** | POTW Water | Deionized Water | ||||
| Conc. (ng/L) | 40 | 160 | 40 | 160 | 40 | 160 | 40 | 160 | 10* (LLOQ) |
| TFA | 106 (16.9) | 97.9 (7.10) | 97.4 (10.8) | 97.6 (6.12) | 97.5 (14.5) | 103 (8.87) | 102 (17.1) | 96.4 (7.33) | 107 (3.55) |
| PFPrA | 95.1 (4.08) | 105 (3.48) | 94.5 (6.85) | 104 (2.36) | 103 (9.37) | 105 (8.34) | 91.8 (4.90) | 104 (7.09) | 109 (1.61) |
| PFBA | 106 (6.80) | 117 (3.18) | 105 (7.40) | 114 (4.91) | 111 (2.48) | 120 (3.27) | 106 (6.58) | 114 (4.85) | 104 (4.91) |
| PFHxA | 93.3 (7.41) | 111 (2.61) | 91.8 (11.34) | 103 (4.55) | 102 (6.62) | 109 (7.11) | 103 (8.37) | 108 (3.13) | 115 (1.64) |
| PFOA | 100 (4.24) | 107 (3.14) | 103 (6.71) | 105 (2.64) | 92.6 (3.85) | 107 (3.09) | 102 (4.57) | 109 (3.64) | 106 (3.28) |
| HFPO-DA | 95.7 (11.9) | 108 (9.05) | 86.6 (8.97) | 104 (5.45) | 94.1 (18.6) | 105 (9.35) | 95.2 (8.49) | 106 (9.23) | 102 (16.8) |
| ADONA | 106 (3.75) | 116 (2.38) | 100 (6.86) | 110 (4.59) | 104 (4.91) | 113 (5.23) | 111 (5.26) | 115 (2.65) | 105 (4.76) |
| PFEtS | 94.8 (9.68) | 110 (5.39) | 89.4 (7.43) | 102 (9.76) | 96.5 (4.09) | 108 (6.11) | 104 (8.18) | 109 (5.23) | 99.8 (9.85) |
| PFPrS | 104 (4.97) | 115 (4.19) | 95.0 (3.87) | 107 (4.26) | 106 (10.6) | 114 (3.36) | 111 (4.88) | 114 (2.96) | 108 (3.28) |
| PFBS | 97.4 (10.1) | 113 (3.97) | 93.6 (5.24) | 104 (4.19) | 97.8 (4.47) | 107 (4.23) | 94.1 (10.7) | 108 (4.48) | 100 (11.0) |
| PFHxS | 99.4 (15.7) | 114 (3.56) | 94.3 (9.79) | 104 (5.28) | 95.2 (5.63) | 112 (3.20) | 104 (8.19) | 111 (4.07) | 107 (11.7) |
| PFOS | 104 (7.54) | 107 (7.69) | 103 (8.43) | 105 (7.23) | 97.3 (14.9) | 110 (4.84) | 109 (7.47) | 108 (7.53) | 102 (4.20) |
| 9Cl-PF3ONS | 98.7 (3.52) | 105 (8.35) | 91.8 (7.66) | 103 (5.68) | 94.7 (9.83) | 105 (8.90) | 105 (6.76) | 107 (8.27) | 107 (4.31) |
| 11Cl-PF3OUdS | 106 (10.1) | 113 (3.54) | 95.0 (3.52) | 113 (8.15) | 107 (6.61) | 112 (4.54) | 119 (4.25) | 120 (9.10) | 98.2 (11.3) |
*20 ng/L LLOQ for TFA
**Groundwater was diluted fivefold for TFA only
Conclusion
A simplified isocratic method was developed and verified for ultrashort-chain PFAS analysis along with alternative and legacy compounds in water samples. Due to the balanced, multimode retention of these analytes on a Raptor Polar X (2.7 µm) 50 x 2.1 mm column, the analytical method was demonstrated to be fast, rugged, and sensitive with acceptable accuracy and precision. This method is suitable for analytical labs wanting to expand their existing PFAS assays for potable or non-potable water to include C2 and C3 compounds.
References
- S. Taniyasu, K. Kannan, L.W.Y. Yeung, K.Y. Kwok, P.K.S Lam, N. Yamashita, Analysis of trifluoroacetic acid and other short-chain perfluorinated acids (C2-C4) in precipitation by liquid chromatography-tandem mass spectrometry: comparison to patterns of long-chain perfluorinated acids (C5-C18), Anal. Chim. Acta. 619 (2008) 221-230. https://pubmed.ncbi.nlm.nih.gov/18558116/
- J. Janda, K. Nodler, H-J. Brauch, C. Zwiener, F.T. Lange, Robust trace analysis of polar (C2-C8) perfluorinated carboxylic acids by liquid chromatography-tandem mass spectrometry: method development and application to surface water, groundwater, and drinking water, Environ. Sci. Pollut.R. 26 (2018) 7326-7336. https://pubmed.ncbi.nlm.nih.gov/29557039/
- K.Y. Kwok, S. Taniyasu, L.W.Y. Yeung, M.B. Murphy, P.K.S. Lam, Y. Horii, K. Kannan, G. Petrick, R.K. Sinha, N. Yamashita, Flux of perfluorinated chemicals through wet deposition in Japan, the United States, and other countries, Environ. Sci. Technol. 44 (2010) 7043-7049. https://pubmed.ncbi.nlm.nih.gov/20795671/