Introduction
Gabapentin is an anticonvulsant drug. This compound is a structural analog of the neurotransmitter gamma-aminobutyric acid (GABA).1 While the primary use of gabapentin is in the treatment of neuropathic pain and seizures, it is commonly prescribed for many off-label uses as well. These include the treatment of anxiety disorders, chronic pain disorders, and restless leg syndrome.2,3

Gabapentin is prescribed in high doses relative to other therapeutic drugs and is eliminated in urine predominantly in its original form.1 Due to the high dosages, and the fact that the chemical structure is not modified by any metabolic processes, detection of extremely high concentrations of this compound in patient urine samples is common when analyzed by drug testing assays. Heltsley et al. (2011) reported the mean concentration of gabapentin to be 430.9 µg/mL in patient urine specimens.1 When analyzed by LC-MS/MS, high concentrations of gabapentin can present significant analytical complications. In this work, we will discuss the analytical challenges associated with high concentrations of gabapentin in urine samples and explore several strategies to address them.
Analytical Challenges of High Gabapentin Levels
Detector Saturation
In targeted mass spectrometry (MS), the detector measures the abundance of ions at selected mass-to-charge ratios (m/z). Saturation of the detector occurs when the intensity of those ions exceeds the upper limit of the detection system. When an analyte saturates the detector, a distorted, flat-topped peak shape is typically displayed in the software. Saturation prevents accurate quantitation of the analyte as the detector response is cut off when it exceeds the upper limit, and the reading is not accurate to what is present in the sample. For samples containing high levels of gabapentin, detector saturation is a concern.
Column Overload
HPLC columns also have limits to analyte concentrations that can be injected while still achieving normal peak shapes due to mass overload. The stationary phase of an HPLC column has a limited number of interaction sites for solute molecules. When there is a greater number of solute molecules seeking interaction sites than are available on the stationary phase, the molecules will continue through the column in the mobile phases until available interaction sites are reached. As analytes move down the column, the sample will be spread across a wider area, which will result in distorted, asymmetric peaks and shifting retention times. High concentrations of gabapentin may cause column overloading and result in unfavorable peak shapes.
Interference
Detector and column overloading of an analyte can negatively affect performance of other nearby eluting compounds. Analytes eluting in the same area as the highly concentrated analyte may suffer from poor peak shape, signal suppression, and retention time shifts. An interference between amphetamine and gabapentin when analyzed by LC-MS/MS has been well documented.4,5 Gabapentin and amphetamine will elute at very similar retention times under certain chromatographic conditions. This may result in signal suppression and shifting retention times for amphetamine when gabapentin is present in high concentrations.
Experimental
In this section, urine samples spiked with various concentrations of gabapentin and amphetamine were analyzed using an LC-MS/MS method (Method 1) developed for the analysis of 60 drugs of abuse in urine. Samples were fortified with varying amounts of gabapentin and amphetamine in urine as described in Table I. The data was examined to determine how analyte performance was impacted by high levels of gabapentin when analyzed by Method 1. Based on these results, a new method (Method 2) was developed to mitigate the analytical challenges presented by samples with high gabapentin concentrations.
Table I: Analyte Concentrations of Samples 1, 2, and 3 in Urine
| Sample | Gabapentin Concentration (µg/mL) | Amphetamine Concentration (µg/mL) |
| 1 | 0.1 | 0.1 |
| 2 | 250 | 0.1 |
| 3 | 500 | – |
Method 1 Instrument Conditions
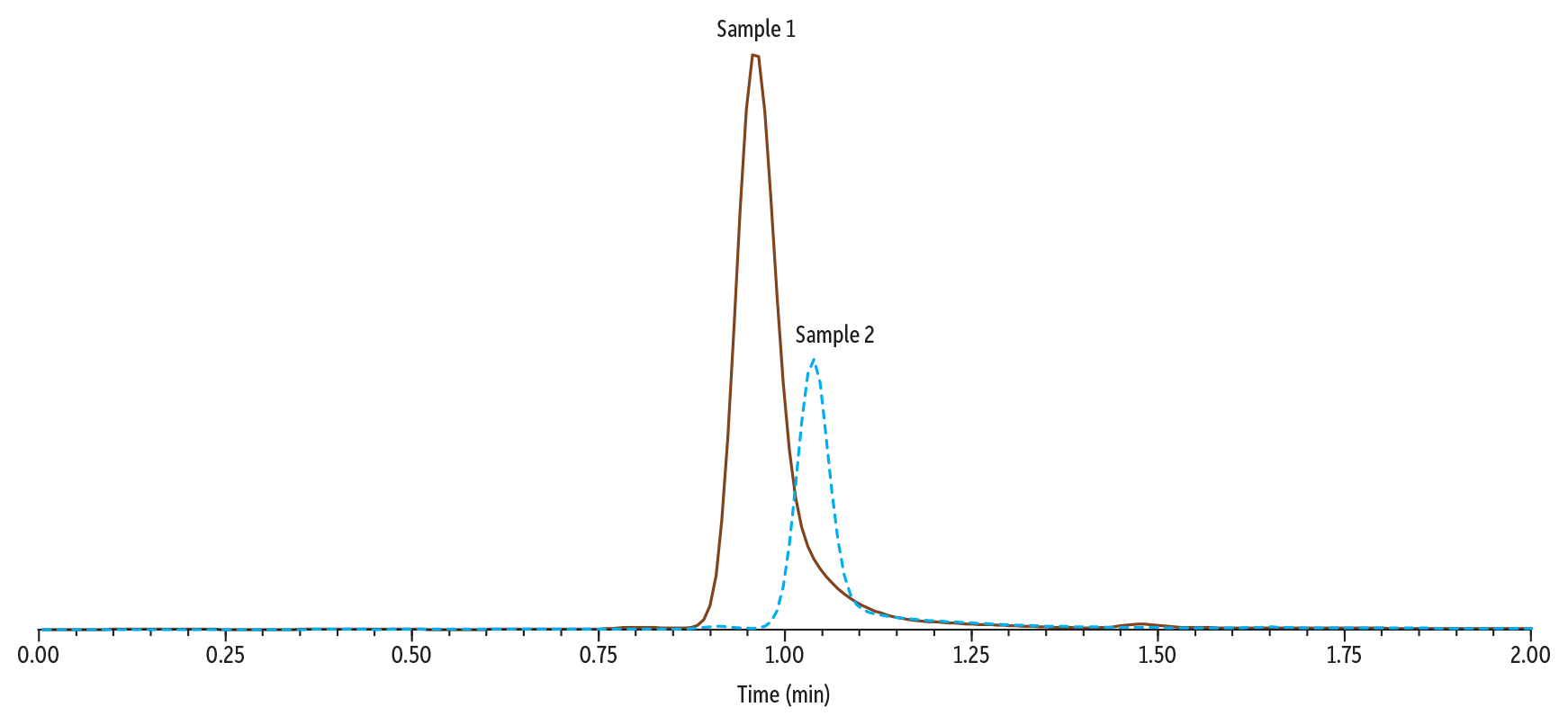
The instrument conditions and an example chromatogram containing all target analytes in Method 1 are shown in Figure 2, below. Under these conditions, gabapentin and amphetamine elute at similar retention times, with gabapentin at 1.09 minutes and amphetamine at 1.03 minutes.
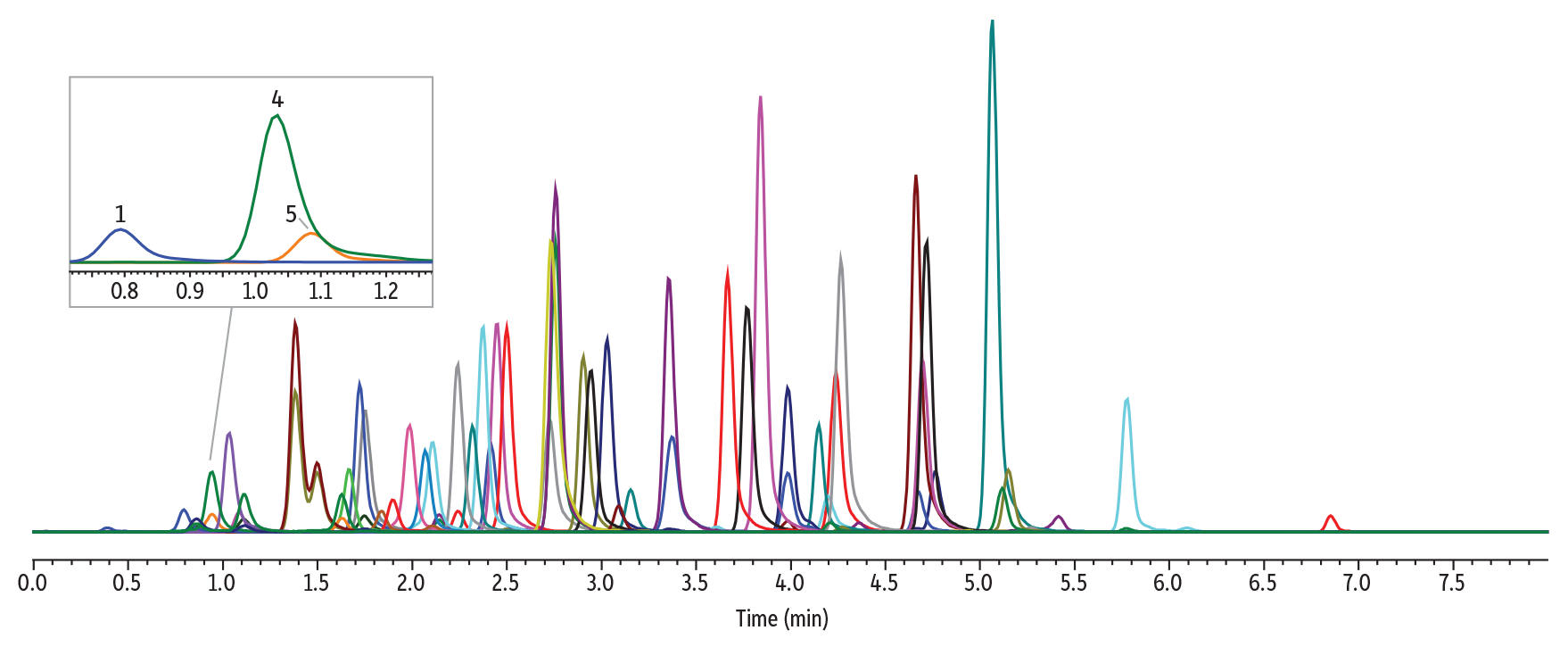
LC_CF0833
Peaks
| Peaks | tR (min) | Precursor | Product 1 | Product 2 | |
|---|---|---|---|---|---|
| 1. | Pregabalin | 0.80 | 160.1 | 142.1 | 55.0 |
| 2. | Morphine | 0.85 | 285.9 | 152.0 | 165.1 |
| 3. | Oxymorphone | 0.95 | 302.0 | 284.1 | 227.0 |
| 4. | Amphetamine | 1.03 | 135.9 | 91.1 | 65.1 |
| 5. | Gabapentin | 1.09 | 172.1 | 154.1 | 136.9 |
| 6. | Hydromorphone | 1.11 | 286.2 | 185.1 | 157.0 |
| 7. | Methamphetamine | 1.38 | 150.1 | 91.2 | 119.0 |
| 8. | Phentermine | 1.50 | 150.1 | 91.2 | 133.2 |
| 9. | Noroxycodone | 1.63 | 301.9 | 227.0 | 197.1 |
| 10. | Naloxone | 1.66 | 327.9 | 310.1 | 212.1 |
| 11. | O-Desmethyltramadol | 1.72 | 250.1 | 58.1 | – |
| 12. | Norhydrocodone | 1.75 | 285.9 | 199.1 | 128.1 |
| 13. | MDMA | 1.75 | 194.1 | 162.9 | 134.9 |
| 14. | Codeine | 1.84 | 300.1 | 165.1 | 215.0 |
| 15. | 6-Acetylmorphine | 1.89 | 328.0 | 165.1 | 211.1 |
| 16. | Oxycodone | 1.97 | 315.9 | 298.1 | 241.1 |
| 17. | Naltrexone | 2.06 | 342.1 | 324.1 | 267.1 |
| 18. | Hydrocodone | 2.10 | 300.1 | 199.1 | 171.1 |
| 19. | Desmethylvenlafaxine | 2.14 | 264.0 | 58.1 | 107.0 |
| 20. | 6-B-Naltrexol | 2.23 | 344.0 | 326.1 | 308.2 |
| 21. | Ritalinic acid | 2.32 | 220.1 | 84.1 | 55.9 |
| 22. | N-Desmethyltapentadol | 2.37 | 208.1 | 107.0 | 121.0 |
| 23. | Norketamine | 2.41 | 223.9 | 125.0 | 89.2 |
| 24. | Hydroxybupropion | 2.44 | 256.1 | 238.0 | 138.9 |
| 25. | Norfentanyl | 2.49 | 233.1 | 84.0 | 55.1 |
| 26. | 4′-Hydroxy nitazene | 2.73 | 369.1 | 100.2 | 72.0 |
| 27. | 7-Hydroxyquetiapine | 2.73 | 399.9 | 269.0 | 208.0 |
| 28. | Tramadol | 2.75 | 264.1 | 58.0 | – |
| 29. | Normeperidine | 2.91 | 233.9 | 160.1 | 56.0 |
| 30. | Benzoylecgonine | 2.95 | 290.1 | 168.1 | 77.0 |
| Peaks | tR (min) | Precursor | Product 1 | Product 2 | |
|---|---|---|---|---|---|
| 31. | Zolpidem phenyl-4-carboxylic acid | 3.03 | 337.9 | 265.1 | 219.2 |
| 32. | Meprobamate | 3.08 | 219.1 | 158.1 | 55.0 |
| 33. | 7-Aminoclonazepam | 3.15 | 286.0 | 121.1 | 249.9 |
| 34. | Venlafaxine | 3.36 | 278.0 | 260.2 | 121.0 |
| 35. | Mirtazapine | 3.37 | 266.1 | 195.1 | 106.9 |
| 36. | Norbuprenorphine | 3.61 | 414.0 | 101.1 | 222.9 |
| 37. | LSD | 3.67 | 324.1 | 223.1 | 208.1 |
| 38. | 9-Hydroxyrisperidone | 3.77 | 427.1 | 207.1 | 110.0 |
| 39. | Acetyl fentanyl | 3.84 | 322.9 | 188.2 | 105.0 |
| 40. | Citalopram | 3.98 | 324.9 | 109.1 | 233.8 |
| 41. | Desmethyldoxepin | 3.99 | 266.0 | 115.0 | 107.0 |
| 42. | Trazodone | 4.14 | 372.1 | 176.1 | 148.0 |
| 43. | Haloperidol | 4.20 | 376.9 | 123.1 | 95.0 |
| 44. | Dextromethorphan | 4.21 | 272.0 | 215.1 | 171.1 |
| 45. | PCP | 4.25 | 244.2 | 86.1 | 159.2 |
| 46. | Fentanyl | 4.26 | 337.0 | 188.0 | 105.1 |
| 47. | Norfluoxetine | 4.27 | 296.0 | 134.0 | 29.9 |
| 48. | Buprenorphine | 4.37 | 468.2 | 55.0 | 396.1 |
| 49. | EDDP | 4.66 | 278.1 | 234.1 | 249.1 |
| 50. | Nortriptyline | 4.68 | 264.1 | 91.2 | 115.0 |
| 51. | Cyclobenzaprine | 4.69 | 276.0 | 215.0 | 189.1 |
| 52. | Sufentanil | 4.72 | 387.0 | 238.0 | 110.9 |
| 53. | Amitriptyline | 4.77 | 278.0 | 202.1 | 91.2 |
| 54. | Methadone | 5.05 | 310.0 | 265.1 | 105.0 |
| 55. | Lorazepam | 5.12 | 320.8 | 275.0 | 302.9 |
| 56. | Oxazepam | 5.15 | 287.0 | 241.0 | 268.5 |
| 57. | Dehydro aripiprazole | 5.27 | 446.0 | 285.1 | 98.1 |
| 58. | α-hydroxyalprazolam | 5.41 | 325.0 | 297.0 | 216.0 |
| 59. | Temazepam | 5.78 | 301.0 | 255.0 | 282.5 |
| 60. | Δ9-THC-COOH | 6.85 | 345.1 | 327.0 | 299.2 |
Conditions
| Column | Raptor Biphenyl (cat.# 9309A52) | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 50 mm x 2.1 mm ID | ||||||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||||||
| Guard Column: | Raptor Biphenyl EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9309A0252) | ||||||||||||||||||||||||||||
| Temp.: | 45 °C | ||||||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||||||
| Diluent: | 90:10 Water:methanol, both with 0.1% formic acid | ||||||||||||||||||||||||||||
| Conc.: | 500 ng/mL | ||||||||||||||||||||||||||||
| Inj. Vol.: | 5 µL | ||||||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||||||
| A: | Water, 0.1% formic acid | ||||||||||||||||||||||||||||
| B: | Methanol, 0.1% formic acid | ||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
| Max Pressure: | 300 bar |
| Detector | Shimadzu 8045 LC-MS/MS |
|---|---|
| Ion Mode: | ESI+ |
| Mode: | MRM |
| Instrument | Shimadzu Nexera X2 |
| Sample Preparation | Control urine (20 µL) was added to a 1.5 mL microcentrifuge tube along with 20 µL of a premade enzyme hydrolysis master mix. The sample was vortexed for 10 seconds and left to incubate at room temperature for 20 minutes. After the incubation, 260 µL of the diluent (water, 0.1 % formic acid:methanol, 0.1 % formic acid 90:10 [v/v]) was added. A 100 µL aliquot was added to a vial insert (cat.# 21776) in a 2.0 mL, amber, short-cap vial (cat.# 21142) and capped with a 9 mm short cap (cat.# 24497) and injected on the LC-MS/MS for analysis. |
Method 1 Results
Amphetamine
In Figure 3, Sample 1 (0.1 µg/mL of gabapentin and amphetamine) and Sample 2 (250 µg/mL of gabapentin and 0.1 µg/mL of amphetamine) were analyzed using Method 1. When compared, the peak height and area for amphetamine are significantly lower in Sample 2 than in Sample 1. This indicates that, under these conditions, the high concentration of gabapentin present in Sample 2 is significantly suppressing the signal of amphetamine. Additionally, in Sample 2, amphetamine experienced a shifted retention time.
Gabapentin
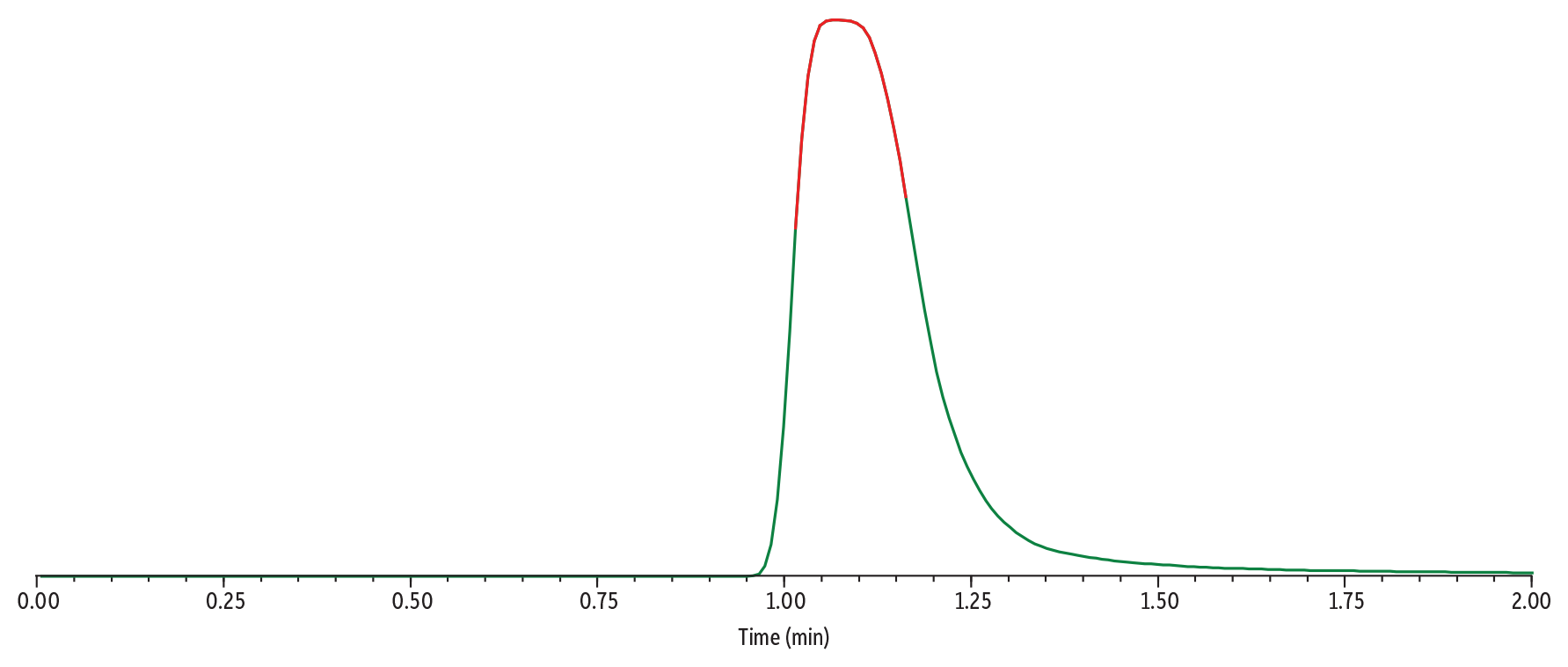
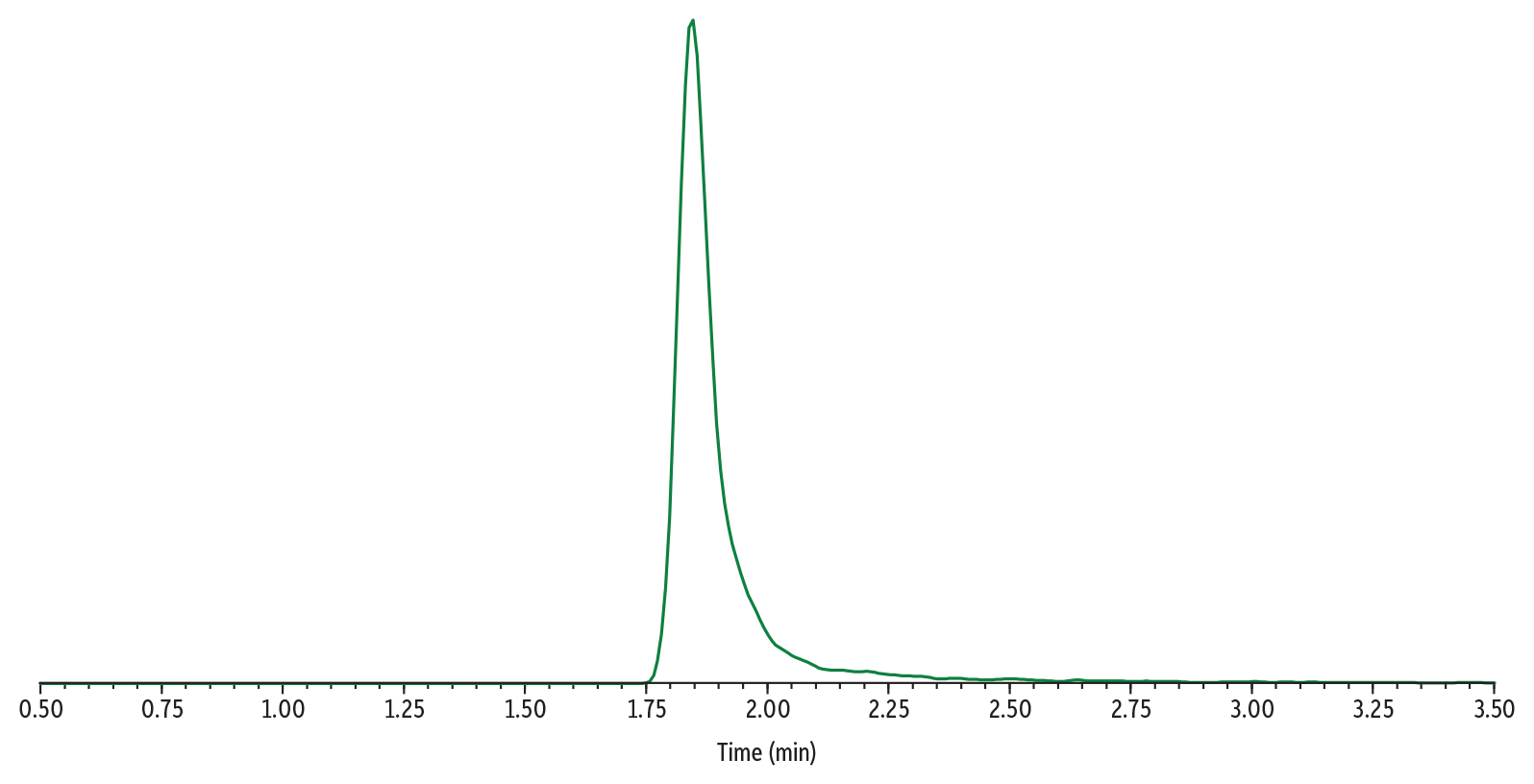
In Figure 4, Sample 3 (500 µg/mL of gabapentin) was analyzed using Method 1. The high concentration of gabapentin results in a wide peak that is tailing significantly. The apex of the peak is beginning to flatten, indicating that the detector is being overloaded.
Method 2 Instrument Conditions
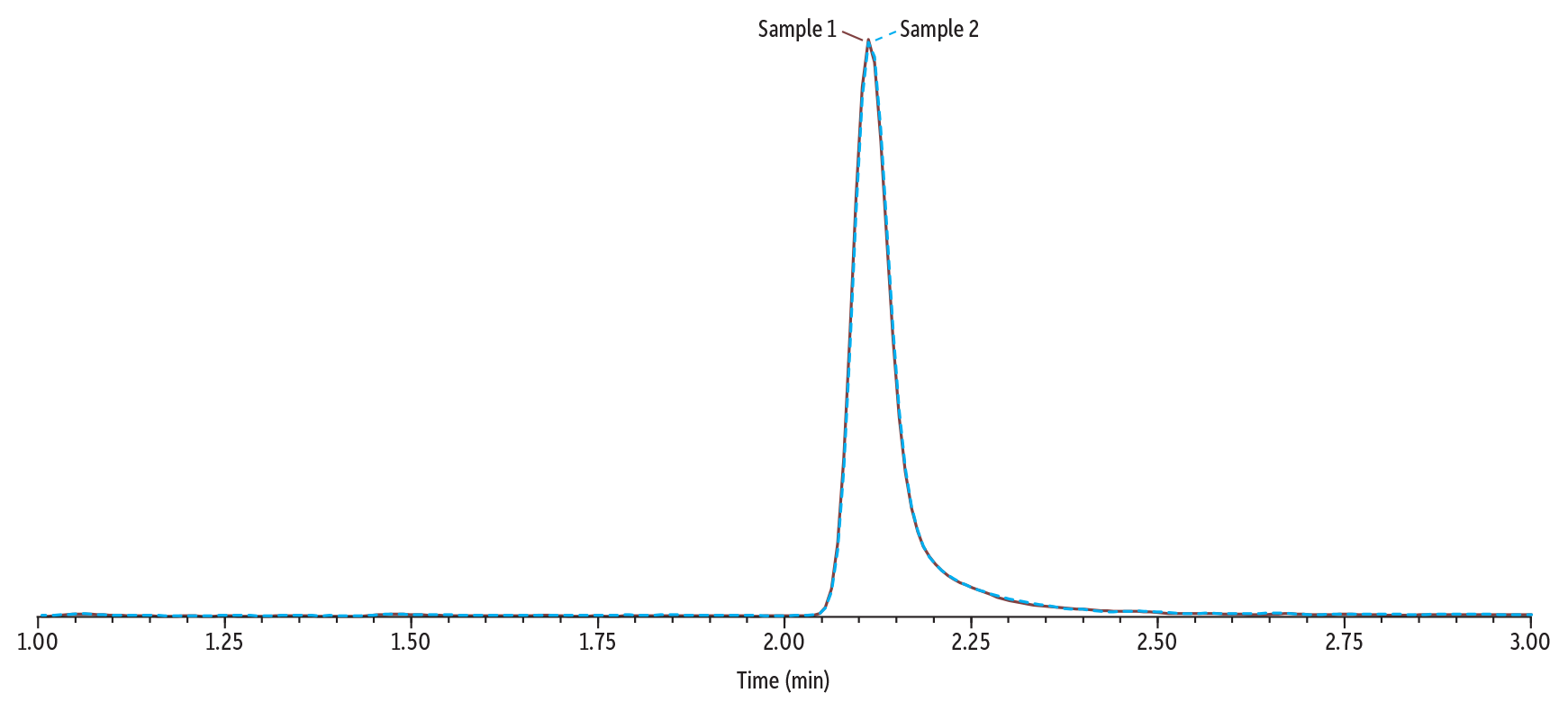
The instrument conditions and an example chromatogram containing all target analytes in Method 2 are shown in Figure 5, below. Under these conditions, gabapentin and amphetamine are chromatographically resolved, with gabapentin at 1.53 minutes and amphetamine at 2.12 minutes.
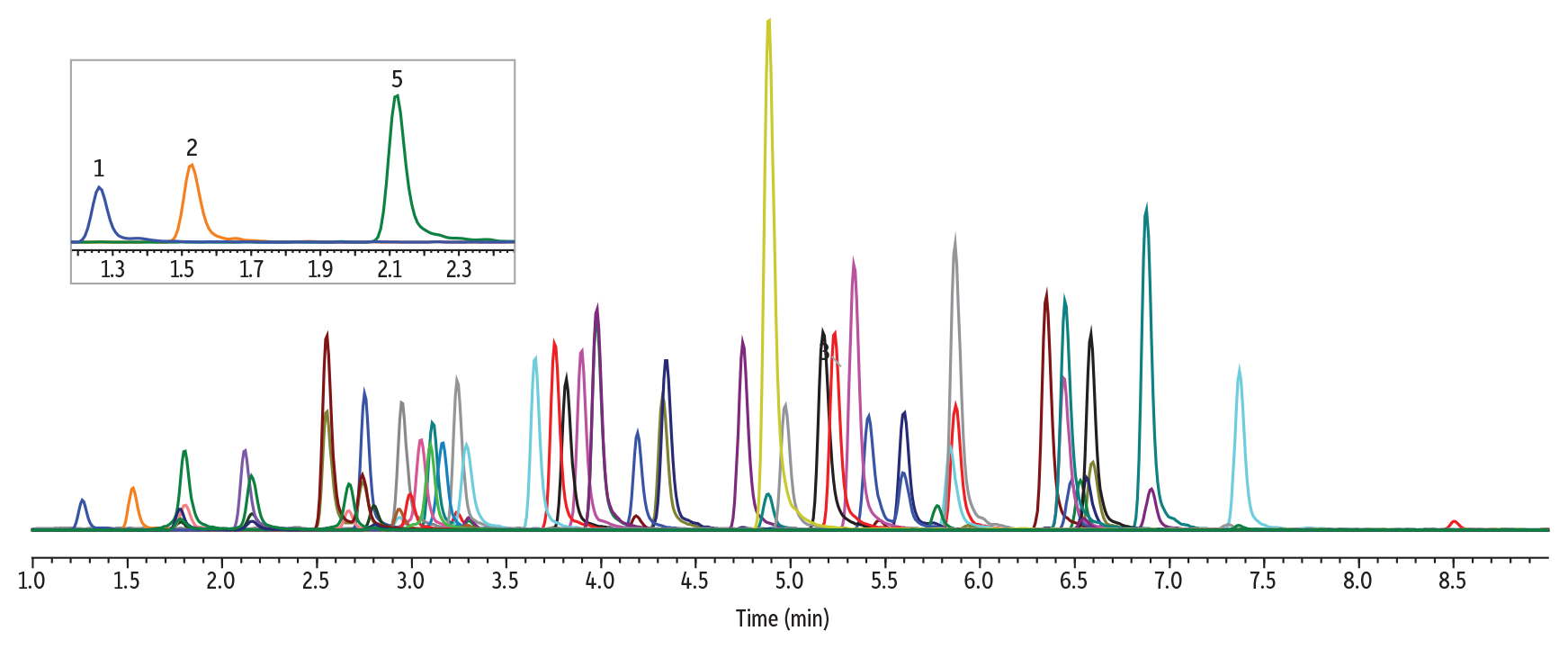
LC_CF0834
Peaks
| Peaks | tR (min) | Precursor | Product 1 | Product 2 | |
|---|---|---|---|---|---|
| 1. | Pregabalin | 1.26 | 160.1 | 142.1 | 55.0 |
| 2. | Gabapentin | 1.53 | 172.1 | 154.1 | 136.9 |
| 3. | Morphine | 1.78 | 285.9 | 152.0 | 165.1 |
| 4. | Oxymorphone | 1.80 | 302.0 | 284.1 | 227.0 |
| 5. | Amphetamine | 2.12 | 135.9 | 91.1 | 65.1 |
| 6. | Hydromorphone | 2.15 | 286.2 | 185.1 | 157.0 |
| 7. | Methamphetamine | 2.55 | 150.1 | 91.2 | 119.0 |
| 8. | Noroxycodone | 2.66 | 301.9 | 227.0 | 197.1 |
| 9. | Phentermine | 2.74 | 150.1 | 91.2 | 133.2 |
| 10. | O-Desmethyltramadol | 2.75 | 250.1 | 58.1 | – |
| 11. | Norhydrocodone | 2.80 | 285.9 | 199.1 | 128.1 |
| 12. | Codeine | 2.92 | 300.1 | 165.1 | 215.0 |
| 13. | MDMA | 2.95 | 194.1 | 162.9 | 134.9 |
| 14. | 6-Acetylmorphine | 2.96 | 328.0 | 165.1 | 211.1 |
| 15. | Oxycodone | 3.05 | 315.9 | 298.1 | 241.1 |
| 16. | Ritalinic acid | 3.12 | 220.1 | 84.1 | 55.9 |
| 17. | Naloxone | 3.13 | 327.9 | 310.1 | 212.1 |
| 18. | Naltrexone | 3.15 | 342.1 | 324.1 | 267.1 |
| 19. | 6-B-Naltrexol | 3.24 | 344.0 | 326.1 | 308.2 |
| 20. | Hydrocodone | 3.29 | 300.1 | 199.1 | 171.1 |
| 21. | Desmethylvenlafaxine | 3.30 | 264.0 | 58.1 | 107.0 |
| 22. | N-Desmethyltapentadol | 3.65 | 208.1 | 107.0 | 121.0 |
| 23. | Norfentanyl | 3.75 | 233.1 | 84.0 | 55.1 |
| 24. | Benzoylecgonine | 3.82 | 290.1 | 168.1 | 77.0 |
| 25. | Hydroxybupropion | 3.89 | 256.1 | 238.0 | 138.9 |
| 26. | Tramadol | 3.97 | 264.1 | 58.0 | – |
| 27. | Meprobamate | 4.19 | 219.1 | 158.1 | 55.0 |
| 28. | Norketamine | 4.20 | 223.9 | 125.0 | 89.2 |
| 29. | Normeperidine | 4.32 | 233.9 | 160.1 | 56.0 |
| 30. | Zolpidem Phenyl-4-carboxylic acid | 4.34 | 337.9 | 265.1 | 219.2 |
| Peaks | tR (min) | Precursor | Product 1 | Product 2 | |
|---|---|---|---|---|---|
| 31. | Venlafaxine | 4.75 | 278.0 | 260.2 | 121.0 |
| 32. | 7-aminoclonazepam | 4.88 | 286.0 | 121.1 | 249.9 |
| 33. | 4′-Hydroxy nitazene | 4.89 | 369.1 | 100.2 | 72.0 |
| 34. | Norbuprenorphine | 4.92 | 414.0 | 101.1 | 222.9 |
| 35. | 7-Hydroxyquetiapine | 4.96 | 399.9 | 269.0 | 208.0 |
| 36. | 9-Hydroxyrisperidone | 5.17 | 427.1 | 207.1 | 110.0 |
| 37. | LSD | 5.23 | 324.1 | 223.1 | 208.1 |
| 38. | Acetyl fentanyl | 5.34 | 322.9 | 188.2 | 105.0 |
| 39. | Mirtazapine | 5.41 | 266.1 | 195.1 | 106.9 |
| 40. | Citalopram | 5.46 | 324.9 | 109.1 | 233.8 |
| 41. | Desmethyldoxepin | 5.60 | 266.0 | 115.0 | 107.0 |
| 42. | Haloperidol | 5.77 | 376.9 | 123.1 | 95.0 |
| 43. | Dextromethorphan | 5.84 | 272.0 | 215.1 | 171.1 |
| 44. | PCP | 5.86 | 244.2 | 86.1 | 159.2 |
| 45. | Fentanyl | 5.87 | 337.0 | 188.0 | 105.1 |
| 46. | Norfluoxetine | 5.93 | 296.0 | 134.0 | 29.9 |
| 47. | EDDP | 6.35 | 278.1 | 234.1 | 249.1 |
| 48. | Trazodone | 6.45 | 372.1 | 176.1 | 148.0 |
| 49. | Cyclobenzaprine | 6.46 | 276.0 | 215.0 | 189.1 |
| 50. | Nortriptyline | 6.48 | 264.1 | 91.2 | 115.0 |
| 51. | Lorazepam | 6.52 | 320.8 | 275.0 | 302.9 |
| 52. | Buprenorphine | 6.55 | 468.2 | 55.0 | 396.1 |
| 53. | Amitriptyline | 6.56 | 278.0 | 202.1 | 91.2 |
| 54. | Sufentanil | 6.58 | 387.0 | 238.0 | 110.9 |
| 55. | Oxazepam | 6.60 | 287.0 | 241.0 | 268.5 |
| 56. | Methadone | 6.88 | 310.0 | 265.1 | 105.0 |
| 57. | α-Hydroxyalprazolam | 6.90 | 325.0 | 297.0 | 216.0 |
| 58. | Dehydro aripiprazole | 7.31 | 446.0 | 285.1 | 98.1 |
| 59. | Temazepam | 7.36 | 301.0 | 255.0 | 282.5 |
| 60. | Δ9-THC-COOH | 8.50 | 345.1 | 327.0 | 299.2 |
Conditions
| Column | Raptor Biphenyl (cat.# 9309A12) | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||||||
| Guard Column: | Raptor Biphenyl EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9309A0252) | ||||||||||||||||||||||||||||
| Temp.: | 45 °C | ||||||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||||||
| Diluent: | 90:10 Water:mobile phase B | ||||||||||||||||||||||||||||
| Conc.: | 500 ng/mL | ||||||||||||||||||||||||||||
| Inj. Vol.: | 2 µL | ||||||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||||||
| A: | Water, 10 mM ammonium formate | ||||||||||||||||||||||||||||
| B: | 90:10 Methanol:2-propanol (v/v), 0.1% formic acid | ||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
| Max Pressure: | 390 bar |
| Detector | Shimadzu 8045 LC-MS/MS |
|---|---|
| Ion Mode: | ESI+ |
| Mode: | MRM |
| Instrument | Shimadzu Nexera X2 |
| Sample Preparation | Control urine (20 µL) was added to a 1.5 mL microcentrifuge tube along with 20 µL of a premade enzyme hydrolysis master mix. The sample was vortexed for 10 seconds and left to incubate at room temperature for 20 minutes. After the incubation, 260 µL of the diluent (water:mobile phase B [v/v]) was added. A 100 µL aliquot was added to a vial insert (cat.# 21776) in a 2.0 mL, amber, short-cap vial (cat.# 21142) and capped with a 9 mm short cap (cat.# 24497) and injected on the LC-MS/MS for analysis. |
Method 2 Results
Amphetamine
In Figure 6, Sample 1 (0.1 µg/mL of gabapentin and amphetamine) and Sample 2 (250 µg/mL of gabapentin and 0.1 µg/mL of amphetamine) were analyzed using Method 2. When compared, the peak height and area for amphetamine are consistent in Sample 1 and Sample 2 despite the high concentration of gabapentin in Sample 2. This indicates that, under these conditions, gabapentin is sufficiently separated from amphetamine so that its signal is not being suppressed.
Gabapentin
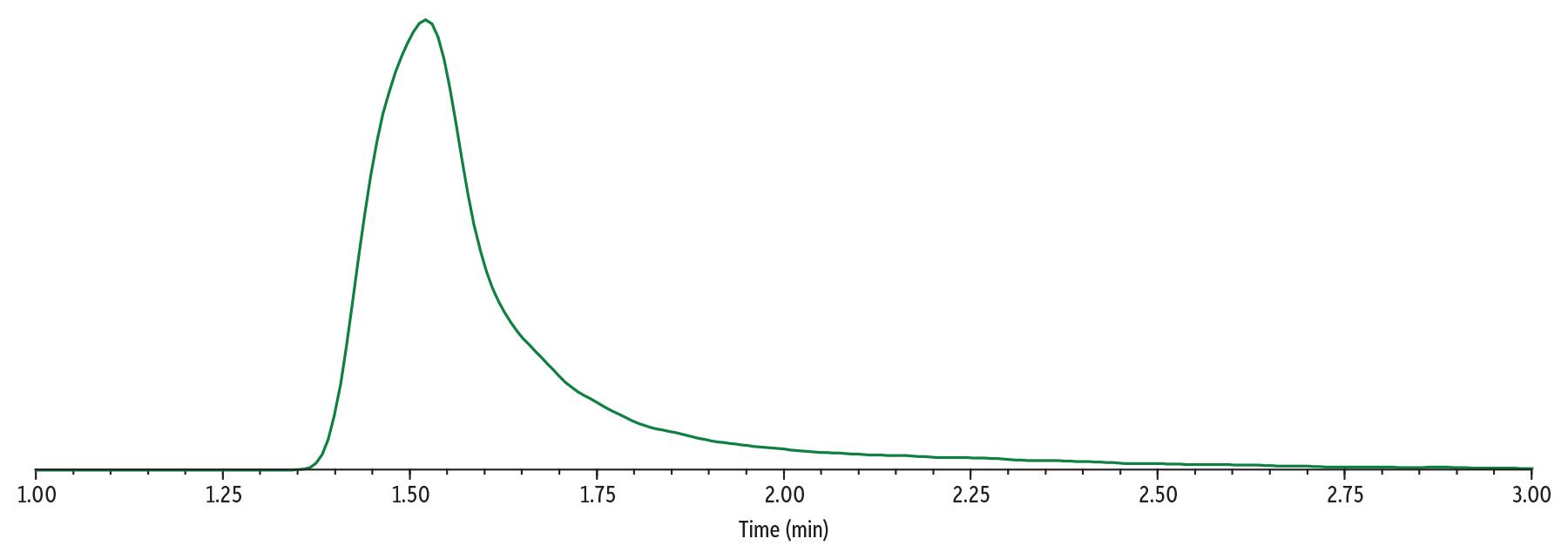
In Figure 7, Sample 3 (500 µg/mL of gabapentin) was analyzed using Method 2. Under these method conditions, the peak shape of gabapentin has improved compared to those used in Method 1 (Figure 4). The peak is not as wide, and the tailing has significantly improved. The collision energy was deoptimized for gabapentin to mitigate detector saturation.
The peak shape of gabapentin can be further improved by moving to a larger-bore column if desired. In Figure 8, 500 µg/mL of gabapentin is shown on a Raptor Biphenyl 50 x 4.6 mm, 2.7 µm column. The flow rate and injection volume have been adjusted to reflect the use of a larger-bore column. While a larger-bore column was not selected for use in the redeveloped method, this is another strategy method developers may employ to further improve the peak performance of gabapentin.
Discussion
Method Development
Method 1 was redeveloped to more effectively handle samples with high levels of gabapentin. Several strategies were employed to accomplish this, such as choosing a longer column length, switching mobile phase additives, and decreasing the injection volume. Carryover was also addressed. These strategies are discussed below.
Column Length
Shorter column lengths, such as 30 or 50 mm, are often preferred as they are capable of shorter runtimes and reduced back pressure. While longer column lengths have more resolving power than shorter columns, they may require longer analysis times and result in higher back pressures. While Method 1 utilized a 50 mm column length, Method 2 used a 100 mm column length. The additional column length allowed for more chromatographic space between gabapentin and amphetamine, which was necessary to reduce suppression in samples with high gabapentin levels.
Mobile Phase Additives
The original method used formic acid as a mobile phase additive. When formic acid was swapped for ammonium formate, the elution order for early eluting compounds was affected. This allowed gabapentin to elute before amphetamine, which successfully mitigated much of the suppression caused by the high concentration of gabapentin.
Injection Volume
As discussed, chromatographic overload is a concern when high concentrations of gabapentin are encountered. One way to mitigate chromatographic overload is by decreasing the sample injection volume. This can also help with detector saturation. For this reason, the injection volume was decreased from 5 µL to 2 µL. This allowed for an improved peak shape for gabapentin even at high concentrations. It is important to consider that lowering the injection volume can raise the limit of detection (LOD) or limit of quantification (LOQ) for other analytes in the method. The performance of the other analytes in the method should be verified to ensure that acceptable LODs/LOQs are still achievable.
Carryover
High concentrations of analyte may result in carryover between samples. 2-Propanol may be added to a mobile phase to help mitigate carryover by washing contaminants off the analytical column more efficiently. For this reason, the composition of Mobile Phase B was altered from 0.1% formic acid in methanol to 0.1% formic acid, 90:10 methanol:2-propanol (v/v).
Deoptimization of MRM Transition
When performing analysis by LC-MS/MS, it is recommended that method developers perform compound tuning to determine the most optimal mass spectrometry parameters for individual analytes. These parameters include precursor/product ions; collision energies; and other voltages. Compound tuning, or optimization, is generally considered good practice as it helps to improve analyte sensitivity. There are unique scenarios, however, in which using non-optimized settings for an analyte may be acceptable. In this work, deoptimization was utilized for gabapentin to mitigate saturation of the mass detector by adjusting settings to reduce the number of product ions hitting the detector. Method developers using deoptimization to help reduce detector saturation should ensure that the necessary detection limits can still be achieved, and that proper identification of the analyte is not affected.
Column Diameter
Although a narrow-bore column (2.1 mm) was chosen for the redeveloped method, a larger-bore (4.6 mm) column was also explored. Larger column IDs offer increased space in the flow path and additional interaction sites for solute molecules to bind to. This can help to mitigate the distorted peak shapes and retention times that result from column overload, which was observed on smaller ID columns. Though the 4.6 mm column ID was successful in improving the peak performance when a large concentration of gabapentin was present, method developers should be aware of the drawbacks that may accompany using a large column ID in this scenario. Larger-bore columns may require an increased injection volume to achieve the same sensitivity as a narrow-bore column. The downside to increasing injection volumes is the increased introduction of matrix on the column that can affect chromatography, decrease column lifetimes, and enhance matrix interferences. The flow rate may also need to be increased to achieve similar analysis times to methods using a narrow-bore column. Increased flow rates may negatively impact ionization efficiency which can reduce sensitivity and require increased solvent usage over narrow-bore columns.
Chromatographic Performance
While the redeveloped method successfully mitigated interference between gabapentin and amphetamine, the performance of the other analytes in the method should also be tested. To ensure that the rest of the analytes still met analysis goals, critical isobars were examined. The reproducibility of the method was also verified by assessing lot-to-lot column variation.
Separation of Isobars
The analyte list contained nine groups of isobaric compounds that share a molecular weight. For quantitative methods to be both precise and rugged, a resolution of 1.5 or greater must be achieved. To ensure that the redeveloped method still achieved adequate resolution of critical isobars, the resolution for each isobar group was calculated (Table IV). All isobar groups had a resolution of 1.5 or better when analyzed using the redeveloped method.
Table II: Compound Name, Shared Molecular Weight, Analyte Retention Time, Peak Width, and Calculated Resolution between Isobar Groups
| Isobar Group | Analyte | Molecular Weight (g/mol) | tr (min) | Peak Width | Resolution |
| 1 | Methamphetamine | 149.2 | 2.55 | 0.106 | 1.7 |
| Phentermine | 2.74 | 0.119 | |||
| 2 | Venlafaxine | 277.4 | 4.75 | 0.113 | 12.2 1.9 |
| EDDP | 6.35 | 0.112 | |||
| Amitriptyline | 6.56 | 0.109 | |||
| 3 | Naloxone | 327.3 | 3.13 | 0.111 | 1.5 |
| 6-Acetylmorphine | 2.96 | 0.107 | |||
| 4 | Morphine | 285.3 | 1.78 | 0.101 | 3.3 5.5 18.6 |
| Hydromorphone | 2.15 | 0.122 | |||
| Norhydrocodone | 2.80 | 0.112 | |||
| 7-amimnoclonazepam | 4.88 | 0.111 | |||
| 5 | Codeine | 299.3 | 2.93 | 0.115 | 2.9 |
| Hydrocodone | 3.29 | 0.126 | |||
| 6 | o-Desmethylvenlaxafine | 263.4 | 3.30 | 0.108 | 6.1 22.6 |
| Tramadol | 3.97 | 0.110 | |||
| Nortriptyline | 6.48 | 0.112 | |||
| 7 | Mirtazapine | 265.3 | 5.41 | 0.125 | 1.6 |
| Desmethyldoxepin | 5.60 | 0.114 | |||
| 8 | Oxymorphone | 301.3 | 1.80 | 0.121 | 7.5 |
| Noroxycodone | 2.66 | 0.107 | |||
| 9 | Citalopram | 324.4 | 5.46 | 0.105 | 12.7 |
| α-Hydroxyalprazolam | 6.90 | 0.121 |
Lot-to-Lot Reproducibility
The chromatographic separation of gabapentin and amphetamine that is featured in the redeveloped method is critical for preventing interference. Given the unique mobile phase composition of Mobile Phase B, the method was tested across three different column lots to ensure that the separation was reproducible. The results of this study are shown in Figure 7. Though minor differences in retention times for both analytes were observed, the chromatographic separation between gabapentin and amphetamine was maintained on all columns. The performance method was consistent across all three lots.
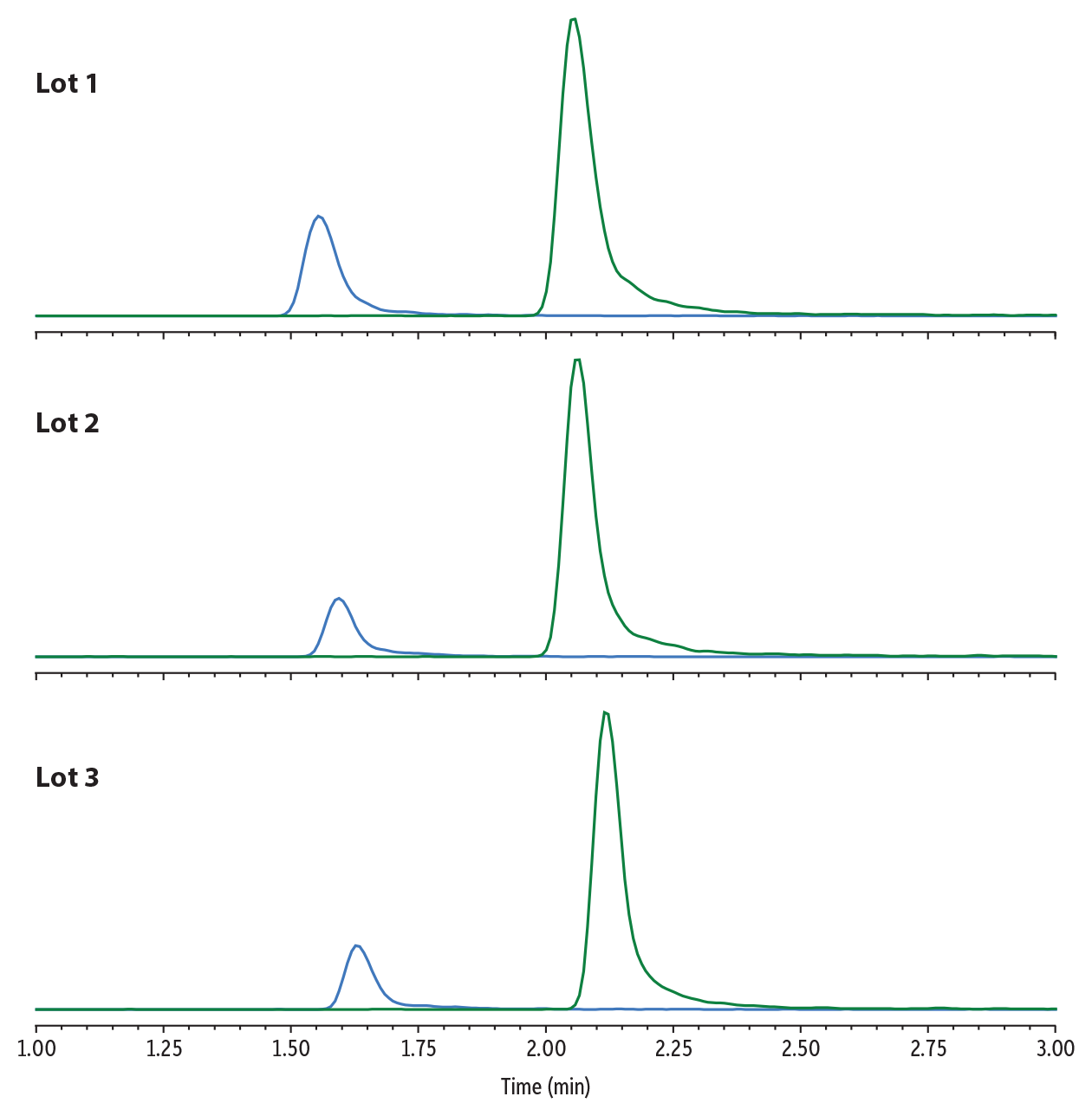
LC_CF0839
Peaks
| Peaks | Lot 1 tR (min) | Lot 2 tR (min) | Lot 3 tR (min) | |
|---|---|---|---|---|
| 1. | Gabapentin | 1.55 | 1.59 | 1.63 |
| 2. | Amphetamine | 2.05 | 2.06 | 2.12 |
Conditions
| Column | Raptor Biphenyl (cat.# 9309A12) | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||||||
| Guard Column: | Raptor Biphenyl EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9309A0252) | ||||||||||||||||||||||||||||
| Temp.: | 45 °C | ||||||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||||||
| Diluent: | 90:10 Water:mobile phase B | ||||||||||||||||||||||||||||
| Inj. Vol.: | 2 µL | ||||||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||||||
| A: | Water, 10 mM ammonium formate | ||||||||||||||||||||||||||||
| B: | 90:10 Methanol:2-propanol (v/v), 0.1% formic acid | ||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
| Max Pressure: | 390 bar |
| Detector | Shimadzu 8045 LC-MS/MS |
|---|---|
| Ion Mode: | ESI+ |
| Mode: | MRM |
| Instrument | Shimadzu Nexera X2 |
| Sample Preparation | Control urine (20 µL) was added to a 1.5 mL microcentrifuge tube along with 20 µL of a premade enzyme hydrolysis master mix. The sample was vortexed for 10 seconds and left to incubate at room temperature for 20 minutes. After the incubation, 260 µL of the diluent (water:mobile phase B [v/v]) was added. A 100 µL aliquot was added to a vial insert (cat.# 21776) in a 2.0 mL, amber, short-cap vial (cat.# 21142) and capped with a 9 mm short cap (cat.# 24497) and injected on the LC-MS/MS for analysis. |
Pregabalin
Pregabalin is another anticonvulsant drug that is structurally related to gabapentin. Like gabapentin, pregabalin is prescribed in very high doses and is eliminated in urine in its original form, which may be detected in urine at elevated concentrations. No specific interference for pregabalin has been widely reported, however, the same analytical complications associated with high levels of gabapentin may also occur for pregabalin due to their structural and metabolic similarities. In the instrument conditions described for Method 2, pregabalin is the first eluting analyte and is well resolved from other early eluting analytes. For samples containing high concentrations of pregabalin, this will help to prevent analyte suppression or retention time shifting of other nearby eluting analytes.
Conclusion
In this work, the effect of high concentrations of gabapentin in urine samples was investigated. An LC-MS/MS method developed for the analysis of 60 drugs of abuse in urine was tested to determine if amphetamine was negatively affected by high levels of gabapentin. The results of these experiments initiated the development of a second method with the purpose of mitigating the impact of high levels of gabapentin. The redeveloped method employed several strategies, including a longer column length, alternative mobile phase composition, and reduced injection volume. The redeveloped method successfully resolved gabapentin from amphetamine, which improved the performance of both analytes. The method is also suitable to handle samples with large concentrations of pregabalin.
Sources
- R. Heltsley, A. Depriest, D. Black, David, T. Robert, Y.H. Caplan, E.J. Cone, Urine drug testing of chronic pain patients. IV. Prevalence of gabapentin and pregabalin. J. Anal. Toxicol. 35 (6) (2011) 357-9. https://doi.org/10.1093/anatox/35.6.357
- A.L. Cross, O. Viswanath, A.L. Sherman, Pregabalin, StatPearls, StatPearls Publishing, Treasure Island, Florida, May 2, 2024, https://www.ncbi.nlm.nih.gov/books/NBK470341/
- R. Yasaei, S. Katta, P. Patel, et al. Gabapentin, StatPearls, StatPearls Publishing, Treasure Island, Florida, Feb 21, 2024 https://www.ncbi.nlm.nih.gov/books/NBK493228/
- S.B. Shugarts, Pervasive gabapentin interference in the LC-MS/MS analysis of amphetamine, JALM 2 (4) (2018) 527–534 https://doi.org/10.1373/jalm.2017.024117
- A.C. Muñoz-Muñoz, T. Pekol, D. Schubring, R. Hyland, C. Johnson, L. Andrade, Characterization of an amphetamine interference from gabapentin in an LC–HRMS method, J. Anal. Toxicol. 44 (1) (2020) 36–40, https://doi.org/10.1093/jat/bkz046
This method has been developed for research use only; it is not suitable for use in diagnostic procedures without further evaluation.