Nitrous oxide (N2O), is commonly known as “laughing” gas, but is also used as a component in fuels in rockets and as an aerosol propellant. N2O is itself a stable gas and can be analyzed relative easy via gas chromatography. Often it is confused with “nitric oxide”, (NO). NO is a very reactive gas. When oxygen is preset, it will immediate oxidize into NO2. NO2 can be easily recognized as it has a dark brown color. Also NO2 shows reactivity, meaning that the analysis of NO and NO2 via gas chromatography is not commonly done.
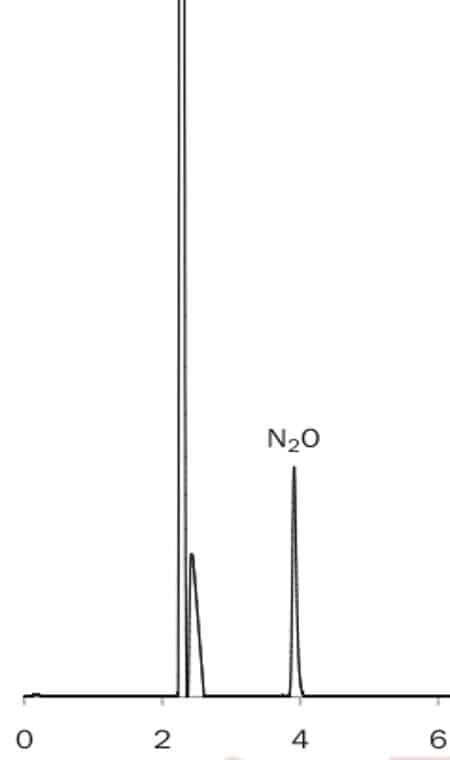
Nitrous oxide on Alumina BOND / Na2SO4 Column: 30m x 0.53mm Rt Alumina BOND / Na2SO4; Helium, 4 mL/min; Oven: 40ºC; sample: 60µl; Detector: PlasmaDetek PED; sample: 5.4 ppm N2O.Chromatogram courtesy: L. Paradis, LDetec.
Recently a summary of N2O analysis was published by Separation science. Separations are shown on different adsorbents like, Porous polymer, Alumina, Molsieve 5A and ShinCarbon materials.
Especially the alumina PLOT is interesting (Fig.1) as often CO2 is present and can interfere with the N2O measurement. CO2 is adsorbed completely by Alumina, resulting is a single N2O peak. CO2 can be removed periodically by conditioning at 200C.


