Key Highlights
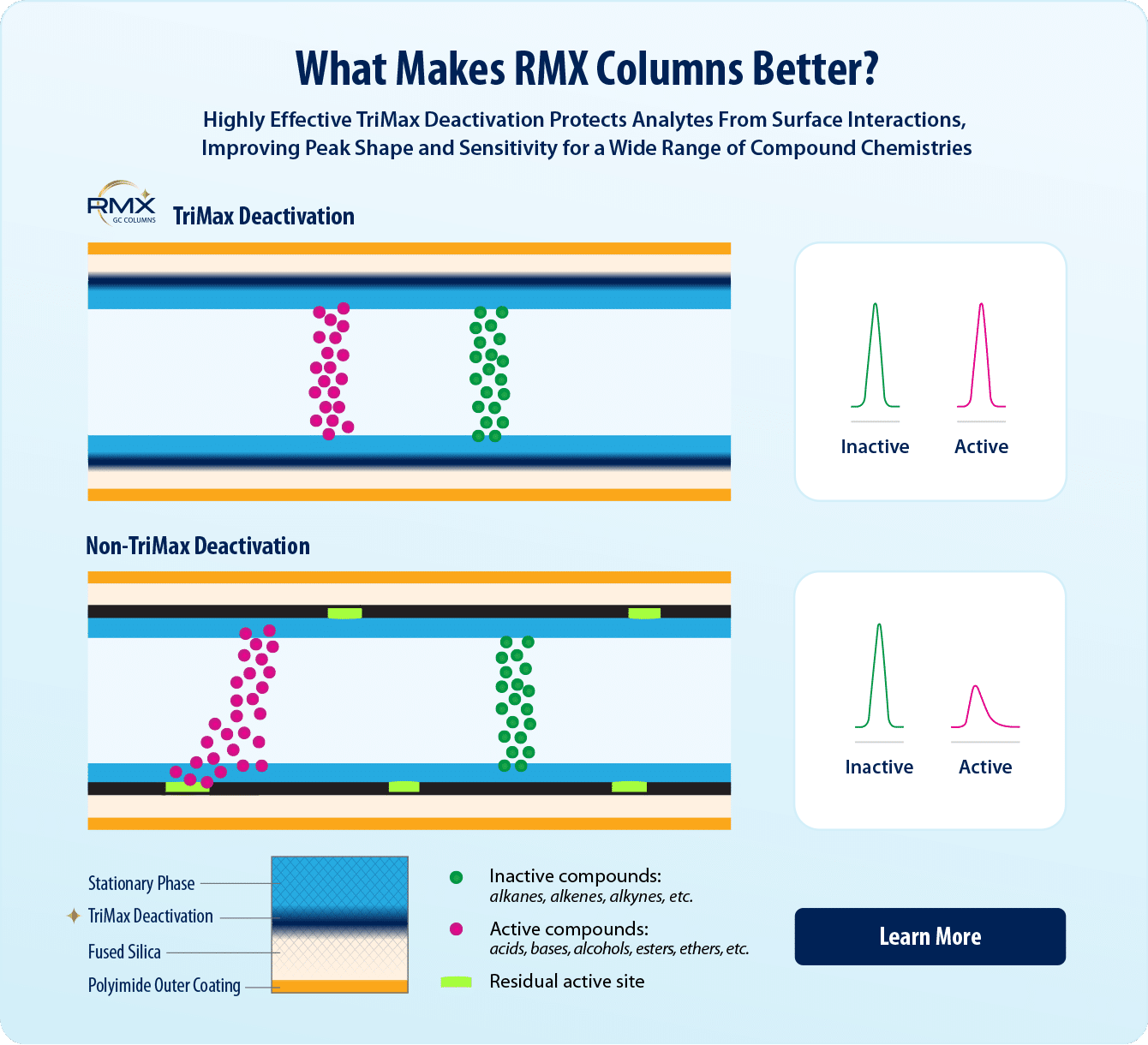
- Next-generation TriMax GC column deactivation creates a robust and exceptionally neutral sample flow path.
- Maximum symmetry reduces peak tailing for a wide range of challenging semivolatiles—including acids, bases, and neutrals—improving identification and quantification.
- Optimized GC-MS conditions speed up sample throughput while still meeting method criteria.

Abstract
RMX-5Sil MS columns feature a groundbreaking deactivation technology that creates a highly inert surface that is broadly effective across a wide range of semivolatile compound classes. In this study, we evaluated the columns performance against the data quality criteria in Method Standard HJ 834-2017. Method criteria for system suitability, linearity, and recovery were easily met with optimized conditions that reduced sample analysis time by 20 minutes.
Introduction
Semivolatile compounds are monitored worldwide to protect human health and the environment. Analysis can be done with a variety of detectors, with GC-MS techniques being among the most popular. The People’s Republic of China Method Standard HJ 834-2017 is used for GC-MS analysis of semivolatiles in soil and sediment samples. To ensure laboratories generate accurate results, Method Standard HJ 834-2017 specifies criteria for key parameters, including system suitability, linearity, and recovery. Since semivolatiles comprise a wide range of compound chemistries, including reactive compounds, it is essential that the sample flow path be highly inert. Activity in the flow path may contribute to poor peak shape and drifting retention times, which can make it difficult to meet data quality requirements.
In this study, we demonstrate how exceptionally inert RMX-5Sil MS columns can help laboratories meet data quality objectives for Method Standard HJ 834-2017 semivolatiles analysis. RMX-5Sil MS columns undergo a unique TriMax surface deactivation that eliminates active sites (e.g., silanols) and is broadly effective across compound classes. The RMX-5Sil MS column contains a traditional 5sil polymer, so it is a direct 5sil replacement, but the highly neutral surface improves peak shape for a wide variety of compounds, making it easier to meet data quality requirements. In this study, in addition to demonstrating the effectiveness of the column for GC-MS semivolatiles analysis, we also developed optimized instrument conditions that speed up run times and improve sample throughput.
Experimental
Standard and Sample Preparation
Multicomponent semivolatiles standards were prepared at 1, 5, 10, 20, and 50 ppm in dichloromethane for calibration. Internal standards and surrogate standards were present at 40 ppm in each calibration standard.
Instrument Conditions
GC-MS semivolatiles analysis was performed following the conditions in Method Standard HJ 834-2017. In addition, semivolatiles analysis was run under optimized conditions to allow faster analysis times. Table I summarizes the instrument conditions for both approaches.
Table I: Instrument Conditions for GC-MS Semivolatiles Analysis
| Parameter | Method Standard HJ 834-2017 Reference Conditions | Optimized Conditions |
|---|---|---|
| Column | RMX-5Sil MS, 30 m x 0.25 mm ID x 0.25 µm (cat.# 17323) | RMX-5Sil MS, 30 m x 0.25 mm ID x 0.25 µm (cat.# 17323) |
| Injection | 1 µL, splitless, 280 °C | 1 µL, splitless, 280 °C |
| Carrier gas | Helium at 1 mL/min (constant flow) | Helium at 1 mL/min (constant flow) |
| Oven | 35 °C (hold 2 min) to 150 °C at 15 °C/min (hold 5 min) to 290 °C at 3 °C/min (hold 2 min) | Faster oven conditions speed up analysis: 35 °C (hold 1 min) to 290 °C at 8 °C/min to 350 °C at 12 °C/min (hold 5 min) |
| Detector | EI; 70 eV; 230 °C source; 150 °C quad; scan 35-450 m/z; solvent delay 5 min; 280 °Ctransfer line | EI; 70 eV; 230 °C source; 150 °C quad; scan 35-450 m/z; solvent delay 2 min; 280 °Ctransfer line |
Results and Discussion
System Suitability
The instrument was tuned with DFTPP in accordance with M0ethod Standard HJ 834-2017, and then system suitability was assessed by evaluating the degradation of DDT to DDE and DDD. The requirement to not exceed 15% was easily met with DDT degradation to both DDE and DDD being <1%.
Chromatographic Performance
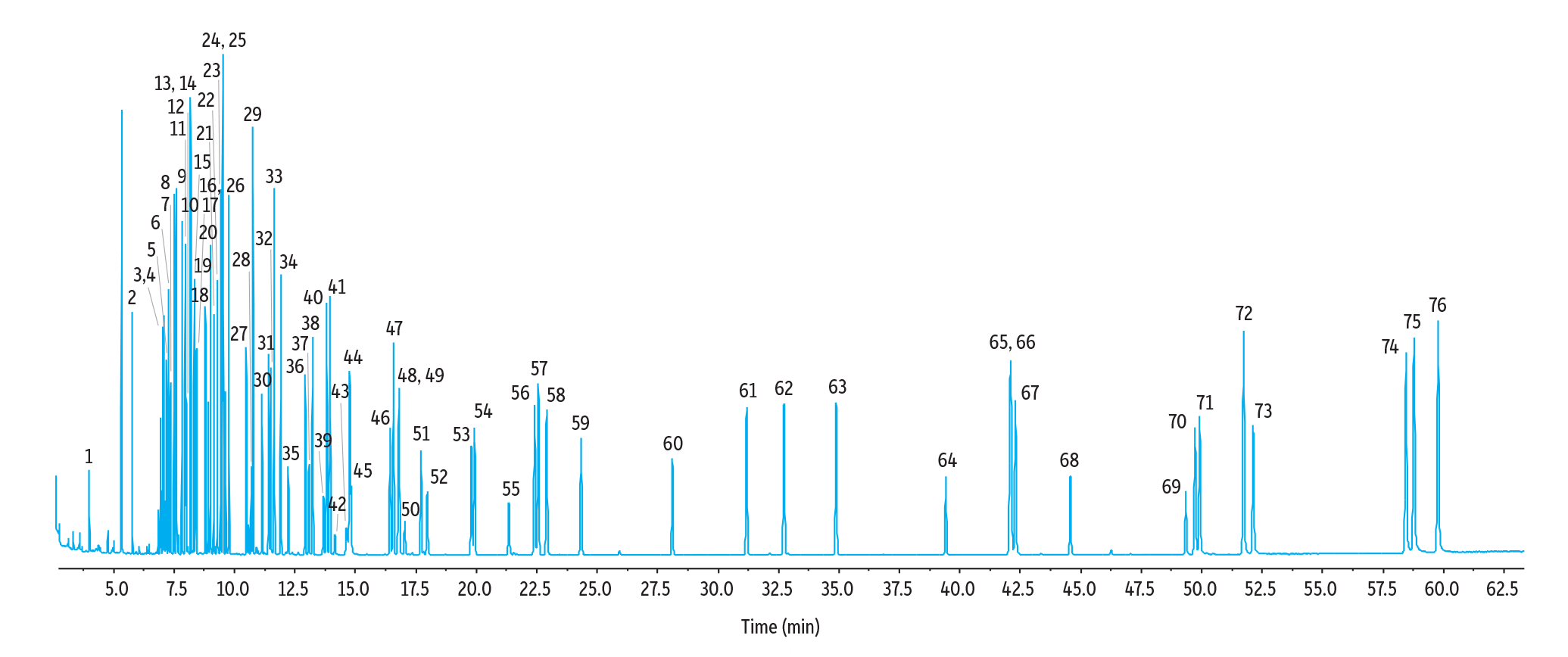
As shown in Figure 1, the RMX-5Sil MS column produced sharp, symmetrical peaks for 64 monitored semivolatiles, six surrogates, and six internal standards (76 compounds total) at 5 ppm under both the original Method Standard HJ 834-2017 conditions and the modified GC-MS conditions. While some coelutions occurred, these compounds could easily be differentiated by their ion m/z ratios. The standard method conditions produced good results, but the >60-minute run time limits the number of samples that can be analyzed. The improved method conditions resulted in a faster 40-minute run time, which is beneficial to high-throughput labs that have many samples to analyze under tight deadlines.
Figure 1: TIC Chromatogram of Method Standard HJ 834-2017 Conditions (>60 minutes)
GC_EV1533
Peaks
| Peaks | tR (min) | |
|---|---|---|
| 1. | Bis(N-methoxy-N-methylamino)methane | 3.989 |
| 2. | Phenol, 2-fluoro- | 5.769 |
| 3. | Phenol-d6- | 7.057 |
| 4. | Phenol | 7.069 |
| 5. | Bis(2-chloroethyl) ether | 7.194 |
| 6. | Phenol, 2-chloro- | 7.269 |
| 7. | Benzene, 1,3-dichloro- | 7.496 |
| 8. | 1,4-Dichlorobenzene-D4 | 7.579 |
| 9. | Benzene, 1,4-dichloro- | 7.602 |
| 10. | Benzene, 1,2-dichloro- | 7.825 |
| 11. | Phenol, 2-methyl- | 7.956 |
| 12. | Bis(2-chloro-1-methylethyl) ether | 7.995 |
| 13. | 1-Propanamine, N-nitroso-N-propyl- | 8.183 |
| 14. | p-Cresol | 8.183 |
| 15. | Ethane, hexachloro- | 8.336 |
| 16. | Nitrobenzene-D5 | 8.393 |
| 17. | Benzene, nitro- | 8.421 |
| 18. | Isophorone | 8.795 |
| 19. | Phenol, 2-nitro- | 8.915 |
| 20. | Phenol, 2,3-dimethyl- | 9.002 |
| 21. | Methane, bis(2-chloroethoxy)- | 9.146 |
| 22. | Phenol, 2,4-dichloro- | 9.290 |
| 23. | Benzene, 1,2,4-trichloro- | 9.427 |
| 24. | Naphthalene-D8 | 9.508 |
| 25. | Naphthalene | 9.540 |
| Peaks | tR (min) | |
|---|---|---|
| 26. | p-Chloroaniline | 9.630 |
| 27. | 1,3-Butadiene, 1,1,2,3,4,4-hexachloro- | 9.767 |
| 28. | Phenol, 2-chloro-5-methyl- | 10.486 |
| 29. | Naphthalene, 1-methyl- | 10.766 |
| 30. | Hexachlorocyclopentadiene | 11.140 |
| 31. | Phenol, 2,4,6-trichloro- | 11.412 |
| 32. | Phenol, 2,4,5-trichloro- | 11.486 |
| 33. | 1,1′-Biphenyl, 2-fluoro- | 11.640 |
| 34. | Naphthalene, 1-chloro- | 11.915 |
| 35. | Dimethyl (2-nitroanilino)maleate | 12.221 |
| 36. | Dimethyl phthalate | 12.926 |
| 37. | Benzene, 2-methyl-1,3-dinitro- | 13.078 |
| 38. | Biphenylene | 13.231 |
| 39. | m-Nitroaniline | 13.680 |
| 40. | Acenaphthene-d10 | 13.813 |
| 41. | Acenaphthene | 13.947 |
| 42. | Phenol, 2,4-dinitro- | 14.149 |
| 43. | Phenol, 4-nitro- | 14.626 |
| 44. | Dibenzofuran | 14.763 |
| 45. | Benzene, 1-methyl-2,4-dinitro- | 14.820 |
| 46. | Diethyl phthalate | 16.434 |
| 47. | Fluorene | 16.576 |
| 48. | Benzene, 1-chloro-4-phenoxy- | 16.802 |
| 49. | Benzene, 1-chloro-3-phenoxy- | 16.802 |
| 50. | Phenol, 2-methyl, 4,6-dinitro- | 17.043 |
| Peaks | tR (min) | |
|---|---|---|
| 51. | Azobenzene | 17.714 |
| 52. | Phenol, 2,4,6-tribromo- | 17.972 |
| 53. | Benzene, 1-bromo-4-phenoxy- | 19.798 |
| 54. | Benzene, hexachloro- | 19.924 |
| 55. | Phenol, pentachloro- | 21.347 |
| 56. | Phenanthrene-D10 | 22.411 |
| 57. | 9H-Fluorene, 9-methylene- | 22.561 |
| 58. | Anthracene | 22.911 |
| 59. | Carbazole | 24.334 |
| 60. | Dibutyl phthalate | 28.107 |
| 61. | Fluoranthene | 31.814 |
| 62. | Pyrene | 32.727 |
| 63. | p-Terphenyl-d14 | 34.881 |
| 64. | Benzyl butyl phthalate | 39.418 |
| 65. | Benz[a]anthracene | 42.103 |
| 66. | Chrysene-D12 | 42.103 |
| 67. | Chrysene | 42.298 |
| 68. | Bis(2-ethylhexyl) phthalate | 44.571 |
| 69. | Phthalic acid, hept-4-yl octyl ester | 49.346 |
| 70. | Benzo[b]fluoranthene | 49.728 |
| 71. | Benzo[k]fluoranthene | 49.915 |
| 72. | Benzo[a]pyrene | 51.738 |
| 73. | Perylene – D12 | 52.132 |
| 74. | Indeno[1,2,3-cd]pyrene | 58.454 |
| 75. | Diben[a,h]anthracene | 58.786 |
| 76. | Benzo[ghi]perylene | 59.786 |
Conditions
| Column | RMX-5Sil MS, 30 m, 0.25 mm ID, 0.25 µm (cat.# 17323) |
|---|---|
| Standard/Sample | |
| 1000 ppm HJ 834-2017 VOC and SVOCs mixture 155 (LGC) | |
| 1000 ppm HJ 834-2017 substitutes mixture 156 (LGC) | |
| 1000 ppm HJ 834-2017 internal standard mixture 174 (internal standard) | |
| Diluent: | Dichloromethane |
| Conc.: | 5 µg/mL |
| Injection | |
| Inj. Vol.: | 1 µL splitless (hold 1 min) |
| Liner: | Topaz 4.0 mm ID single taper inlet liner w/wool (cat.# 23303) |
| Inj. Temp.: | 280 °C |
| Oven | |
| Oven Temp.: | 35 °C (hold 2 min) to 150 °C at 15 °C/min (hold 5 min) to 290 °C at 3 °C/min (hold 2 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 1 mL/min @ 35 °C |
| Detector | MS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mode: | Scan | ||||||||
| Scan Program: | |||||||||
| |||||||||
| Transfer Line Temp.: | 280 °C | ||||||||
| Source Temp.: | 230 °C | ||||||||
| Quad Temp.: | 150 °C | ||||||||
| Electron Energy: | 70 eV | ||||||||
| Tune Type: | PFTBA | ||||||||
| Ionization Mode: | EI | ||||||||
| Instrument | Agilent 7890B GC & 5977B MSD | ||||||||
| Sample Preparation | Reference standards were diluted to 5 ppm in dichloromethane. | ||||||||
Figure 2: TIC Chromatogram of Optimized Method Conditions (40 minutes)
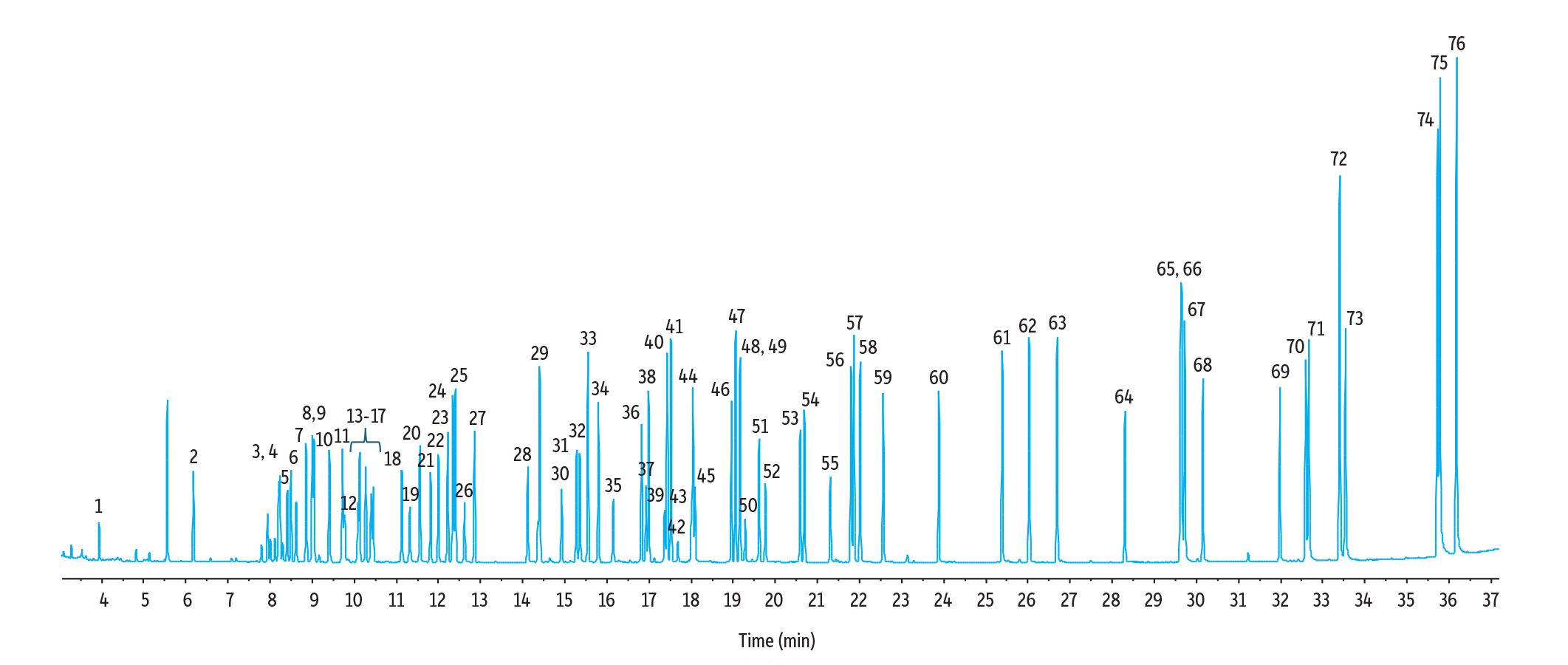
GC_EV1532
Peaks
| Peaks | tR (min) | |
|---|---|---|
| 1. | Bis(N-methoxy-N-methylamino)methane | 3.941 |
| 2. | Phenol, 2-fluoro- | 6.189 |
| 3. | Phenol-d6- | 8.226 |
| 4. | Phenol | 8.252 |
| 5. | Bis(2-chloroethyl) ether | 8.417 |
| 6. | Phenol, 2-chloro- | 8.502 |
| 7. | Benzene, 1,3-dichloro- | 8.861 |
| 8. | 1,4-Dichlorobenzene-D4 | 9.01 |
| 9. | Benzene, 1,4-dichloro- | 9.048 |
| 10. | Benzene, 1,2-dichloro- | 9.411 |
| 11. | Phenol, 2-methyl- | 9.726 |
| 12. | Bis(2-chloro-1-methylethyl) ether | 9.784 |
| 13. | 1-Propanamine, N-nitroso-N-propyl- | 10.101 |
| 14. | p-Cresol | 10.133 |
| 15. | Ethane, hexachloro- | 10.273 |
| 16. | Nitrobenzene-D5 | 10.421 |
| 17. | Benzene, nitro- | 10.468 |
| 18. | Isophorone | 11.137 |
| 19. | Phenol, 2-nitro- | 11.322 |
| 20. | Phenol, 2,3-dimethyl- | 11.561 |
| 21. | Methane, bis(2-chloroethoxy)- | 11.822 |
| 22. | Phenol, 2,4-dichloro- | 12.006 |
| 23. | Benzene, 1,2,4-trichloro- | 12.23 |
| 24. | Naphthalene-D8 | 12.36 |
| 25. | Naphthalene | 12.411 |
| Peaks | tR (min) | |
|---|---|---|
| 26. | p-Chloroaniline | 12.622 |
| 27. | 1,3-Butadiene, 1,1,2,3,4,4-hexachloro- | 12.859 |
| 28. | Phenol, 2-chloro-5-methyl- | 14.122 |
| 29. | Naphthalene, 1-methyl- | 14.409 |
| 30. | Hexachlorocyclopentadiene | 14.927 |
| 31. | Phenol, 2,4,6-trichloro- | 15.284 |
| 32. | Phenol, 2,4,5-trichloro- | 15.362 |
| 33. | 1,1′-Biphenyl, 2-fluoro- | 15.564 |
| 34. | Naphthalene, 1-chloro- | 15.806 |
| 35. | Dimethyl (2-nitroanilino)maleate | 16.158 |
| 36. | Dimethyl phthalate | 16.837 |
| 37. | Benzene, 2-methyl-1,3-dinitro- | 16.945 |
| 38. | Biphenylene | 16.995 |
| 39. | m-Nitroaniline | 17.381 |
| 40. | Acenaphthene-d10 | 17.45 |
| 41. | Acenaphthene | 17.533 |
| 42. | Phenol, 2,4-dinitro- | 17.692 |
| 43. | Phenol, 4-nitro- | 18.011 |
| 44. | Dibenzofuran | 18.05 |
| 45. | Benzene, 1-methyl-2,4-dinitro- | 18.104 |
| 46. | Diethyl phthalate | 18.972 |
| 47. | Fluorene | 19.061 |
| 48. | Benzene, 1-chloro-4-phenoxy- | 19.168 |
| 49. | Benzene, 1-chloro-3-phenoxy- | 19.175 |
| 50. | Phenol, 2-methyl, 4,6-dinitro- | 19.301 |
| Peaks | tR (min) | |
|---|---|---|
| 51. | Azobenzene | 19.626 |
| 52. | Phenol, 2,4,6-tribromo- | 19.78 |
| 53. | Benzene, 1-bromo-4-phenoxy- | 20.596 |
| 54. | Benzene, hexachloro- | 20.697 |
| 55. | Phenol, pentachloro- | 21.316 |
| 56. | Phenanthrene-D10 | 21.822 |
| 57. | 9H-Fluorene, 9-methylene- | 21.88 |
| 58. | Anthracene | 22.025 |
| 59. | Carbazole | 22.57 |
| 60. | Dibutyl phthalate | 23.889 |
| 61. | Fluoranthene | 25.396 |
| 62. | Pyrene | 26.035 |
| 63. | p-Terphenyl-d14 | 26.717 |
| 64. | Benzyl butyl phthalate | 28.309 |
| 65. | Benz[a]anthracene | 29.638 |
| 66. | Chrysene-D12 | 29.684 |
| 67. | Chrysene | 29.744 |
| 68. | Bis(2-ethylhexyl) phthalate | 30.159 |
| 69. | Phthalic acid, hept-4-yl octyl ester | 31.991 |
| 70. | Benzo[b]fluoranthene | 32.608 |
| 71. | Benzo[k]fluoranthene | 32.683 |
| 72. | Benzo[a]pyrene | 33.417 |
| 73. | Perylene – D12 | 33.583 |
| 74. | Indeno[1,2,3-cd]pyrene | 35.743 |
| 75. | Diben[a,h]anthracene | 35.798 |
| 76. | Benzo[ghi]perylene | 36.197 |
Conditions
| Column | RMX-5Sil MS, 30 m, 0.25 mm ID, 0.25 µm (cat.# 17323) |
|---|---|
| Standard/Sample | |
| 1000 ppm HJ 834-2017 VOC and SVOCs mixture 155 (LGC) | |
| 1000 ppm HJ 834-2017 substitutes mixture 156 (LGC) | |
| 1000 ppm HJ 834-2017 internal standard mixture 174 (internal standard) | |
| Diluent: | Dichloromethane |
| Conc.: | 5 µg/mL |
| Injection | |
| Inj. Vol.: | 1 µL splitless (hold 1 min) |
| Liner: | Topaz 4.0 mm ID single taper inlet liner w/wool (cat.# 23303) |
| Inj. Temp.: | 280 °C |
| Oven | |
| Oven Temp.: | 35 °C (hold 1 min) to 290 °C at 8 °C/min to 350 °C at 12 °C/min (hold 5 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 1 mL/min @ 35 °C |
| Detector | MS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mode: | Scan | ||||||||
| Scan Program: | |||||||||
| |||||||||
| Transfer Line Temp.: | 280 °C | ||||||||
| Source Temp.: | 230 °C | ||||||||
| Quad Temp.: | 150 °C | ||||||||
| Electron Energy: | 70 eV | ||||||||
| Tune Type: | PFTBA | ||||||||
| Ionization Mode: | EI | ||||||||
| Instrument | Agilent 7890B GC & 5977B MSD | ||||||||
| Sample Preparation | Reference standards were diluted to 5 ppm in dichloromethane. | ||||||||

Quantitative Performance
Good peak shape improves signal-to-noise ratios, which allows reliable integration at low concentrations and minimizes the need for time-consuming user intervention. Accurate integration across the calibration range increases confidence that method performance criteria will be met, even for difficult reactive compounds. Method performance was assessed by evaluating linearity (unweighted, R>0.990); relative response factor (RSD <30%); and recovery of a midpoint standard (70-130% recovery at 10 ppm).
Limits of detection and quantitation were determined using generally accepted methods where LOD = 3.3 x standard deviation of the lowest calibration point/calibration slope, and LOQ = 10 x standard deviation of the lowest calibration point/calibration slope. Values were compared to the LOD and LOQ values described in the method. The method requirements show LOD and LOQ as sample concentrations. Since matrix was not used in this study, sample concentrations were converted to extract concentrations based on a theoretical sample mass of 20 g, and final extract volume of 1 mL (Table II). Without matrix, the differences between column performance can be separated from the efficacy of the sample preparation.
For quantitative analysis, calibration was performed using the optimized instrument conditions. Summarized results are presented in Table II and the values for individual semivolatiles are given in Table III. All compounds (excluding internal standards and surrogates) passed linearity criteria of RRF RSD <30% and R>0.990. RSD values ranged 2.1-22.5% and R ranged 0.999-1.00. While not required for HJ 834, R2 was also evaluated because it is often employed in other methods. In this study R2 was >0.995 for each compound, ranging 0.997-1.0.
Table II: Results Summary for Method Standard HJ 834-2017 GC-MS Semivolatiles Analysis (Optimized Conditions)
| RRF %RSD (<30%) | R2* (>0.990) | R (>0.990) | LOD (Method HJ 834-2017) | LOQ (Method HJ 834-2017) | %Recovery | |
|---|---|---|---|---|---|---|
| Acceptable | 64 | 64 | 64 | 64 | 64 | 64 |
| Unacceptable | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 23% | 1.000 | 1.000 | 0.89 | 2.98 | 109% |
| Minimum | 2% | 0.997 | 0.999 | 0.02 | 0.05 | 75% |
Table III: Individual Results by Compound for Method Standard HJ 834-2017 GC-MS Semivolatiles Analysis (Optimized Conditions)
| Compound | RRF %RSD (<30%) | R2* | R | Method HJ 834-2017 LOD (ppm) | Method HJ 834-2017 LOQ (ppm) | LOD (ppm) | LOQ (ppm) | %Recovery of 10 ppm |
|---|---|---|---|---|---|---|---|---|
| Bis(N-methoxy-N-methylamino)methane | 2% | 1.000 | 1.000 | 1.60 | 6.40 | 0.36 | 1.22 | 105% |
| Phenol | 4% | 0.998 | 0.999 | 2.00 | 8.00 | 0.33 | 1.11 | 95% |
| Bis(2-chloroethyl) ether | 3% | 0.999 | 1.000 | 2.00 | 8.00 | 0.17 | 0.58 | 99% |
| Phenol, 2-chloro- | 4% | 0.999 | 0.999 | 2.00 | 8.00 | 0.16 | 0.55 | 96% |
| Benzene, 1,3-dichloro- | 5% | 0.999 | 0.999 | 1.80 | 7.20 | 0.20 | 0.68 | 100% |
| Benzene, 1,4-dichloro- | 5% | 1.000 | 1.000 | 1.20 | 4.80 | 0.19 | 0.62 | 97% |
| Benzene, 1,2-dichloro- | 7% | 1.000 | 1.000 | 1.60 | 6.40 | 0.13 | 0.45 | 96% |
| Phenol, 2-methyl- | 5% | 0.998 | 0.999 | 1.60 | 6.40 | 0.25 | 0.82 | 101% |
| Bis(2-chloro-1-methylethyl) ether | 6% | 0.999 | 0.999 | 1.60 | 6.40 | 0.34 | 1.13 | 104% |
| 1-Propanamine, N-nitroso-N-propyl- | 3% | 1.000 | 1.000 | 2.00 | 8.00 | 0.43 | 1.43 | 109% |
| p-Cresol | 6% | 1.000 | 1.000 | 2.00 | 8.00 | 0.11 | 0.38 | 100% |
| Ethane, hexachloro- | 10% | 1.000 | 1.000 | 2.00 | 8.00 | 0.53 | 1.77 | 97% |
| Benzene, nitro- | 7% | 0.999 | 1.000 | 1.40 | 5.60 | 0.23 | 0.76 | 102% |
| Isophorone | 6% | 1.000 | 1.000 | 2.00 | 8.00 | 0.09 | 0.30 | 99% |
| Phenol, 2-nitro- | 10% | 1.000 | 1.000 | 2.00 | 8.00 | 0.15 | 0.50 | 96% |
| Phenol, 2,3-dimethyl- | 6% | 1.000 | 1.000 | 1.80 | 7.20 | 0.25 | 0.84 | 99% |
| Methane, bis(2-chloroethoxy)- | 5% | 1.000 | 1.000 | 1.40 | 5.60 | 0.09 | 0.31 | 100% |
| Phenol, 2,4-dichloro- | 13% | 1.000 | 1.000 | 4.00 | 16.00 | 0.09 | 0.30 | 98% |
| Benzene, 1,2,4-trichloro- | 11% | 1.000 | 1.000 | 1.80 | 7.20 | 0.16 | 0.54 | 98% |
| Naphthalene | 9% | 0.998 | 0.999 | 1.60 | 6.40 | 0.16 | 0.54 | 99% |
| p-Chloroaniline | 8% | 0.999 | 0.999 | 1.40 | 5.60 | 0.17 | 0.57 | 94% |
| 1,3-Butadiene, 1,1,2,3,4,4-hexachloro- | 5% | 1.000 | 1.000 | 1.40 | 5.60 | 0.29 | 0.98 | 97% |
| Phenol, 2-chloro-5-methyl- | 6% | 1.000 | 1.000 | 1.80 | 7.20 | 0.23 | 0.77 | 93% |
| Naphthalene, 1-methyl- | 10% | 0.999 | 0.999 | 1.80 | 7.20 | 0.07 | 0.22 | 98% |
| Hexachlorocyclopentadiene | 22% | 0.998 | 0.999 | 1.20 | 4.80 | 0.27 | 0.92 | 89% |
| Phenol, 2,4,6-trichloro- | 21% | 0.999 | 1.000 | 1.20 | 4.80 | 0.13 | 0.44 | 92% |
| Phenol, 2,4,5-trichloro- | 21% | 0.999 | 0.999 | 1.60 | 6.40 | 0.27 | 0.91 | 92% |
| Naphthalene, 1-chloro- | 15% | 0.999 | 1.000 | 2.00 | 8.00 | 0.10 | 0.32 | 95% |
| Dimethyl (2-nitroanilino)maleate | 21% | 0.999 | 1.000 | 2.00 | 8.00 | 0.62 | 2.05 | 89% |
| Dimethyl phthalate | 15% | 1.000 | 1.000 | 2.00 | 8.00 | 0.12 | 0.39 | 93% |
| Benzene, 2-methyl-1,3-dinitro- | 20% | 0.998 | 0.999 | 2.00 | 8.00 | 0.56 | 1.87 | 90% |
| Biphenylene | 18% | 1.000 | 1.000 | 2.00 | 8.00 | 0.10 | 0.32 | 99% |
| m-Nitroaniline | 22% | 0.999 | 1.000 | 1.60 | 6.40 | 0.89 | 2.98 | 90% |
| Acenaphthene | 10% | 0.998 | 0.999 | 1.80 | 7.20 | 0.02 | 0.08 | 99% |
| Phenol, 2,4-dinitro- | 16% | 0.997 | 0.999 | 1.40 | 5.60 | 0.69 | 2.32 | 75% |
| Phenol, 4-nitro- | 13% | 1.000 | 1.000 | 1.60 | 7.20 | 0.23 | 0.75 | 85% |
| Dibenzofuran | 14% | 1.000 | 1.000 | 2.00 | 8.00 | 0.09 | 0.29 | 99% |
| Benzene, 1-methyl-2,4-dinitro- | 23% | 1.000 | 1.000 | 2.00 | 8.00 | 0.13 | 0.42 | 91% |
| Diethyl Phthalate | 13% | 1.000 | 1.000 | 2.00 | 8.00 | 0.05 | 0.18 | 96% |
| Fluorene | 15% | 0.999 | 1.000 | 1.80 | 7.20 | 0.04 | 0.14 | 98% |
| Benzene, 1-chloro-4-phenoxy- | 8% | 1.000 | 1.000 | 1.80 | 7.20 | 0.12 | 0.39 | 103% |
| Benzene, 1-chloro-3-phenoxy- | 13% | 1.000 | 1.000 | 4.00 | 16.00 | 0.33 | 1.11 | 95% |
| Phenol, 2-methyl, 4,6-dinitro- | 22% | 0.998 | 0.999 | 1.60 | 6.40 | 0.39 | 1.30 | 103% |
| Azobenzene | 6% | 0.999 | 0.999 | 6.00 | 24.00 | 0.15 | 0.50 | 104% |
| Benzene, 1-bromo-4-phenoxy- | 13% | 1.000 | 1.000 | 2.00 | 8.00 | 0.20 | 0.67 | 95% |
| Benzene, hexachloro- | 8% | 1.000 | 1.000 | 2.00 | 8.00 | 0.02 | 0.07 | 99% |
| Phenol, pentachloro- | 11% | 0.998 | 0.999 | 2.00 | 8.00 | 0.39 | 1.30 | 89% |
| 9H-Fluorene, 9-methylene- | 8% | 0.998 | 0.999 | 2.00 | 8.00 | 0.03 | 0.11 | 101% |
| Anthracene | 13% | 1.000 | 1.000 | 4.00 | 16.00 | 0.03 | 0.09 | 104% |
| Carbazole | 11% | 0.998 | 0.999 | 2.00 | 8.00 | 0.07 | 0.24 | 96% |
| Dibutyl phthalate | 12% | 0.999 | 0.999 | 2.00 | 8.00 | 0.03 | 0.10 | 99% |
| Fluoranthene | 10% | 1.000 | 1.000 | 4.00 | 16.00 | 0.07 | 0.22 | 100% |
| Pyrene | 9% | 1.000 | 1.000 | 2.00 | 8.00 | 0.03 | 0.11 | 101% |
| Benzyl butyl phthalate | 12% | 0.998 | 0.999 | 2.00 | 8.00 | 0.20 | 0.68 | 94% |
| Benz[a]anthracene | 5% | 0.999 | 0.999 | 2.00 | 8.00 | 0.08 | 0.26 | 95% |
| Triphenylene | 7% | 0.999 | 1.000 | 2.00 | 8.00 | 0.13 | 0.42 | 106% |
| Bis(2-ethylhexyl) phthalate | 15% | 1.000 | 1.000 | 4.00 | 16.00 | 0.09 | 0.29 | 102% |
| Phthalic acid, hept-4-yl octyl ester | 18% | 1.000 | 1.000 | 2.00 | 8.00 | 0.02 | 0.05 | 103% |
| Benzo[b]fluoranthene | 17% | 0.999 | 1.000 | 2.00 | 8.00 | 0.09 | 0.31 | 96% |
| Benzo[k]fluoranthene | 16% | 1.000 | 1.000 | 4.00 | 16.00 | 0.09 | 0.32 | 102% |
| Benzo[a]pyrene | 15% | 0.999 | 0.999 | 2.00 | 8.00 | 0.08 | 0.26 | 104% |
| Indeno[1,2,3-cd]pyrene | 11% | 0.999 | 1.000 | 2.00 | 8.00 | 0.05 | 0.18 | 96% |
| Diben[a,h]anthracene | 5% | 0.999 | 0.999 | 2.00 | 8.00 | 0.10 | 0.35 | 107% |
| Benzo[ghi]perylene | 7% | 1.000 | 1.000 | 4.00 | 16.00 | 0.04 | 0.15 | 106% |
Conclusion
This study demonstrated that the highly inert RMX-5Sil MS column produced method-compliant results for a wide range of challenging semivolatiles. The inertness of the column surface produced sharp, symmetrical peaks that simplified integration and resulted in excellent results for system suitability; linearity (RRF %RSD and R2); LOD; LOQ; and recovery. In addition to meeting Method Standard HJ 834-2017 requirements and producing highly linear calibration curves, the optimized GC-MS conditions that were developed here reduced sample analysis time from 60 to 40 minutes, allowing high-throughput laboratories to improve sample throughput.