Cannabis testing labs continue to experience serious pressure to provide low-cost, quick turnaround, and favorable cannabinoid reporting capabilities. Some clients prefer labs that report the highest cannabinoid and tetrahydrocannabinol (THC) potency results to drive up the cost of goods at retail sale. The higher the THC content, the more expensive the commodity. Producers and processors may send samples to multiple labs, and then select the lab providing the highest THC results. Unfortunately, in some cases, this has caused labs to report inflated THC numbers and sacrifice scientific integrity to retain their customer base and attract new customers. This practice has coined the phrase “lab shopping.”
To remedy this situation, many have pressured government agencies, such as the Department of Cannabis Control (DCC), to address unethical reporting and establish regulations around testing. But ultimately, solving this problem comes back to the science behind the numbers, and the ability of a lab to select the best method that produces valid results. With many sample extraction methods to choose from, labs may have difficulty determining which procedure to use. To complicate the decision further, many labs also require cost-effective, validated methods with minimal sample touch time.
In this study, we evaluated several cannabinoid extraction methods to help labs establish potency testing methods that will provide the best return on investment without sacrificing data quality. Six extraction methods that are commonly used for plant and flower material were compared in terms of overall extraction efficiency, solvent cost, consumables cost, and sample preparation time.
Experimental Design and Sample Extraction Methods
Two types of chemovars were used, CBD-dominant and CBG-dominant, to ensure extraction efficiency is consistent across different varieties. A total of 16 cannabinoids were monitored for this study. Two Restek certified reference materials (cannabinoids acids 7 standard [cat. # 34114] and cannabinoids neutrals 9 standard [cat.# 34132]) were used to prepare calibration curves from 1 ppm to 500 ppm.
Six extraction methods were chosen for evaluation based upon both national recognition and the recommendations and/or requirements of specific states. The full preparatory procedures for the extractions can be found in Table I. Protocols were modified as needed to match equipment capability, to obtain a consistent dilution of 10- and 100-fold, and to prevent diluent/mobile phase mismatch.
Table I: Prescribed procedures for six cannabinoid extraction methods.
| Method (Reference) | Method 11 | Method 22 | Method 33 |
| Homogenized Flower (mg) | 200 | 500 | 500 |
| Solvent Type | Methanol | Ethanol | Ethanol |
| Solvent Amount (mL) | 20 | 50 | 50 |
| Workflows | Weigh 200 mg flower/leaf cutting into a 50 mL centrifuge tube. Homogenize using ceramic homogenizers and a commercial grinder. Add 20 mL of methanol. Vortex/shake for 10 minutes (A 100-fold dilution). Aliquot 1 mL into a new vial. Centrifuge at 5000 rpm for five minutes. Transfer 50 µL of the supernatant to a new vial. Add 950 µL methanol. Mix briefly (20-fold dilution for a total dilution of 2000-fold). Filter with 4 mm, 0.45 µm regenerated cellulose (RC) syringe filters. | Homogenize 5 g hemp flower (particle size ≤1 mm). Note: Frozen ballmilling is the preferred method of homogenization without sample degradation. Low temperature homogenization prevents degradation of analytes and produces uniform particle sizes. It is essential to ensure the sampling protocol adheres to local guidelines and provides an accurate representation of the bulk material. Weigh 0.5 ±0.01 g on a calibrated microbalance, and transfer sample into a 50 mL polypropylene centrifuge tube. Dispense 20 mL of ethanol into the tube and vortex briefly; then incubate sample on horizontal shaker for 30 minutes at 250 rpm. Centrifuge sample at 4000 rpm for 5 minutes to pellet plant material. Carefully pour the supernatant into an amber 50 mL volumetric flask and set aside for second extraction. Perform second extraction of material with 20 mL ethanol and add extract to an amber 50 mL volumetric flask containing contents of the first extraction. Fill flask to the mark with ethanol and mix well. Perform 1:10 and 1:100 dilution of sample with methanol. Filter samples directly into HPLC vials with 0.2 µm PTFE membrane. | Weigh 0.5 g ± 0.01 g of a thoroughly homogenized sample into a 50 mL centrifuge tube. Add 20 mL of ethanol, briefly shake by hand or mix with a vortex mixer, and then shake for 30 minutes using a horizontal shaker set at approximately 250 rpm. Centrifuge the tube >3000 g for 5 minutes, and filter the supernatant through filter paper into a 50 mL volumetric flask. Transfer sample material back into a 50 mL tube and repeat steps (2) and (3) Note: Collect the supernatant from the second extraction into the same 50 mL volumetric flask. Dilute flask to volume with ethanol. Filter a 3 mL aliquot of extract using a plastic syringe fitted with a 0.22 µm PTFE syringe filter into a 15 mL centrifuge tube. Perform 10- and 100-fold dilution of the extract with methanol in a 10 mL volumetric flask. Note: higher dilution factors can be used if required. Transfer aliquots of the original extract and diluted extracts into a 2 mL amber LC vials, cap, and briefly mix with a vortex mixer. |
| Method (Reference) | Method 44 | Method 55 | Method 6 |
| Homogenized Flower (mg) | 100 | 200 | 500 |
| Solvent Type | Methanol:water (80:20) | Acetonitrile:methanol (80:20) | Methanol |
| Solvent Amount (mL) | 10 | 40 | 10 |
| Workflows | Weigh 100 mg ±5 mg of sample into 15 mL tubes, recording the mass to an accuracy of 0.1 mg. Add 5 mL ±0.1 mL methanol:water (80:20, v:v). Vortex at high speed for 90 seconds ±10 seconds. Centrifuge at 5000 rpm ±500 rpm (4700 ±470 rcf) for 5 ±0.5 minutes. Transfer the supernatant to clean 15 ml tubes. Add a second 5 mL ±0.1 mL aliquot of methanol:water (80:20, v:v) to the 15 mL tubes containing the cannabis matrix sample. Vortex at high speed for 90 seconds ±10 seconds. Centrifuge at 5000 rpm ±500 rpm for 5 minutes ±0.5 minutes. Transfer the supernatant to the same 15 mL tube containing the supernatant from the first extraction. Vortex the supernatant for 5-10 seconds. | Add 40 mL extraction solvent to a 50 mL centrifuge tube containing 200 mg flower sample. For plant material, use acetonitrile:methanol (80:20) as the extraction solvent. Vortex each centrifuge tube for 1 minute to mix the sample and extraction solvent well. Extract in a sonicating bath for 30 minutes with ice in the water bath. Centrifuge at 3900 rpm for 15 minutes. Take approximately 1.5 mL of the supernatant and filter through a 0.2 µm PTFE filter into an HPLC vial. Dilute the sample extract so the expected concentration will be within the range of the calibration curve. | Weigh 500 mg homogenized flower into a 15 mL centrifuge tube. Add 5 mL methanol. Vortex 15 seconds and sonicate 2.5 minutes (x3). Centrifuge at 4000 rpm for 5 minutes. Pour the supernatant into a clean 15 mL centrifuge tube. Repeat steps 2-4 Transfer the supernatant to the same tube containing supernatant from the first extraction. Vortex briefly. Dilute the supernatant 10- and 100-fold with water:acetonitrile (25:75). Filter using a 0.2 µm filter vial. |
Extraction Solvent Cost
Once the extraction methods were chosen, solvent costs were calculated. To determine the solvent cost per mL, the cost of each extraction solvent was calculated using an average list price across varying grades, including LC-MS and HPLC grades. Then, the extraction solvent cost per sample was calculated by multiplying the average solvent cost per mL by the solvent volume required for each extraction method. The results are captured in Table II.
Table II: Solvent cost calculated per sample for each extraction method. Method 5 was the most expensive and Method 6 was the least expensive.
| Extraction Method | Solvent Volume (mL) | Solvent Type | Solvent Cost / mL | Extraction Solvent Cost / Sample |
| Method 1 | 20 | Methanol | $ 0.038 | $ 0.77 |
| Method 2 | 50 | Ethanol | $ 0.045 | $ 2.26 |
| Method 3 | 50 | Ethanol | $ 0.045 | $ 2.26 |
| Method 4 | 8 | Methanol | $ 0.038 | $ 0.42 |
| 2 | Water | $ 0.057 | ||
| Method 5 | 32 | Acetonitrile | $ 0.108 | $ 3.76 |
| 8 | Methanol | $ 0.038 | ||
| Method 6 | 10 | Methanol | $ 0.038 | $ 0.38 |
Extraction Consumables Cost
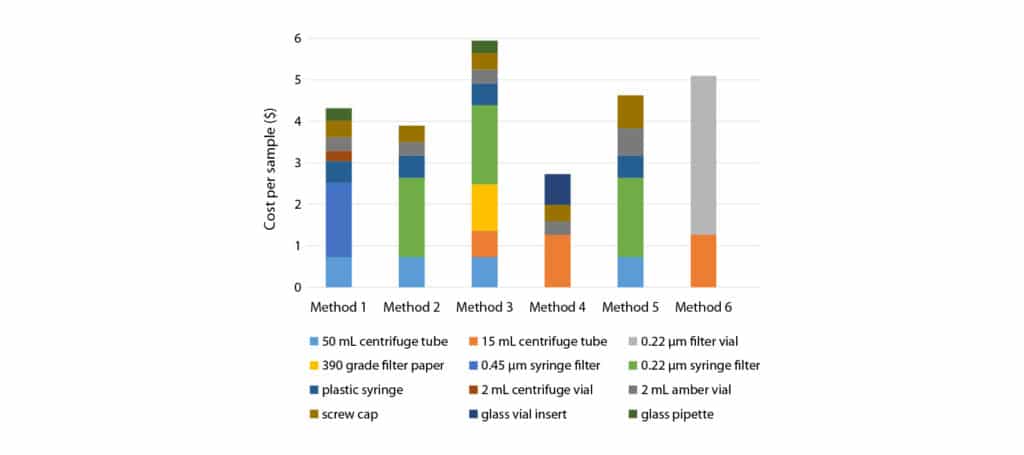
For each method, the total consumables cost per sample was calculated by adding the cost of all consumables used to extract a sample. The average list price across varying qualities/grades was used for each product. Results are captured in Figure 1.
Figure 1: Calculated consumable cost for each cannabinoid extraction method.
Total Cost per Sample
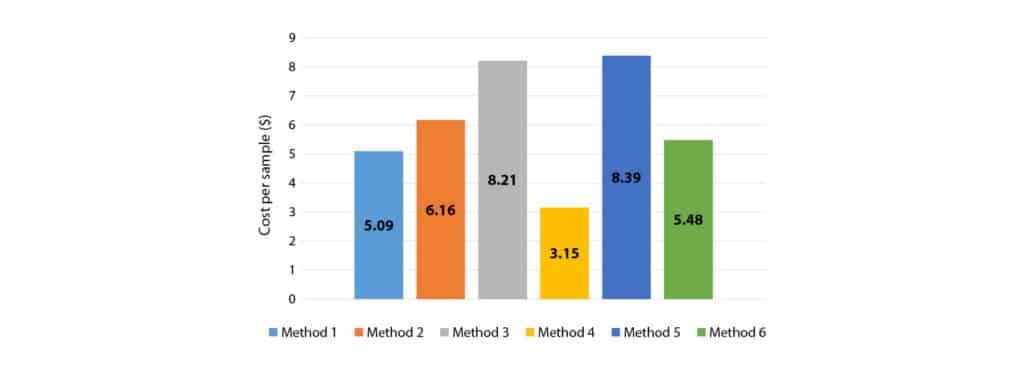
The total cost of extracting a sample was calculated by adding the solvent cost and consumables cost per sample, Table II and Figure 1 respectively, and the results shown in Figure 2.
Figure 2: Calculated total cost per sample for cannabinoid extraction using each procedure.
Preparation of Extractions and Calibration Curves
All results in this study were obtained using CBD-dominant and CBG-dominant chemovars. The traditional method to determine extraction efficiency for naturally occurring compounds is to use a standard addition approach, but in this case the concentrations of the cannabinoids are too high to use this approach. Instead, extraction efficiency was calculated by performing a second solvent extraction for each procedure. The first extraction was carried out as prescribed in Table I, with the modifications as previously stated. Upon completion of the first extraction, the supernatant was poured into a centrifuge tube. This extract was analyzed as a concentrated solution for the minor cannabinoids and diluted 10- and 100-fold for the major cannabinoids. The remainder of the matrix and extract was vacuum filtered through a 0.22 µm filter membrane. Once dried, the sample was transferred to an appropriately sized, clean centrifuge tube, and the extraction procedure was repeated. The second extract was analyzed as a concentrated sample.
Calibration curves (1–500 ppm) for 16 cannabinoids were prepared using two certified reference materials (CRM) from Restek: the cannabinoids acids 7 standard and the cannabinoids neutrals 9 standards. Use of these standards provided a significant reduction in calibration curve preparation time and minimized the potential for preparation errors. The extracts from each procedure were then analyzed using the conditions and materials in Table III.
Table III: Conditions used for analysis of sample extracts from each preparation method.
| Column: | Raptor ARC-18, 2.7 µm 150 mm x 4.6 mm (cat.# 9314A65) | |
| Guard: | Raptor ARC-18, 2.7 µm 5 mm x 4.6 mm ID (cat.# 9314A0250) | |
| Standards: | Cannabinoids acids 7 (cat.# 34144) and cannabinoids neutrals 9 (cat.# 34132) | |
| Diluent: | 25:75 Water:acetonitrile | |
| Inj. Vol. | 5 µL | |
| Mobile phase A | 5 mM Ammonium formate + 0.1% formic acid in water | |
| Mobile phase B | 0.1% Formic acid in acetonitrile | |
| Flow: | 1.5 mL/min | |
| Detector | UV/Vis @ 228 nm | |
| Temp: | 30 °C | |
| Gradient: | Time (min.) | % B |
| 0.00 | 75 | |
| 9.00 | 75 | |
Cannabinoid Content Results
The total cannabinoid content was calculated for the first and second cannabinoid extractions using the following formulas. Each extraction method was tested across two chemovar types: CBG dominant (chemovar 1) and CBD dominant (chemovar 2). Results for the CBG dominant chemovar are captured in Tables IV and V. Results for the CBD dominant chemovar are captured in Tables VI and VII.
Formula 1:
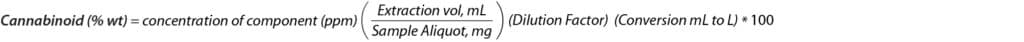
Formula 2:

Formula 3:

Formula 4:

Table IV: Calculated cannabinoid content in first extracts using chemovar #1 (CBG dominant).
| Analyte | Method 1 | Method 2 | Method 3 | C of A | ||||
| mg/g | wt % | mg/g | wt % | mg/g | wt % | mg/g | wt % | |
| Cannabidivarinic acid (CBDVA) | – | ND | – | ND | – | ND | – | ND |
| Cannabidivarin (CBDV) | 0.49 | 0.05 | – | ND | – | ND | – | ND |
| Cannabidiolic acid (CBDA) | – | ND | – | ND | – | ND | – | ND |
| Cannabigerolic acid (CBGA) | 73.47 | 7.35 | 66.66 | 6.67 | 45.36 | 4.54 | 113.50 | 11.35 |
| Cannabigerol (CBG) | 147.44 | 14.74 | 20.46 | 2.05 | 13.56 | 1.36 | 6.00 | 0.60 |
| Cannabidiol (CBD) | 16.58 | 1.66 | – | ND | – | ND | 0.80 | 0.08 |
| Tetrahydrocannabivarin (THCV) | 0.36 | 0.04 | 0.18 | 0.02 | 0.13 | 0.01 | – | ND |
| Tetrahydrocannabivarinic acid (THCVA) | 0.23 | 0.02 | – | ND | – | ND | – | ND |
| Cannabinol (CBN) | – | ND | – | ND | – | ND | – | ND |
| Cannabinolic acid (CBNA) | – | ND | – | ND | – | ND | – | ND |
| Delta 9 Tetrahydrocannabinol (∆9-THC) | 0.78 | 0.08 | 0.35 | 0.03 | 0.27 | 0.03 | – | ND |
| Delta 8 Tetrahydrocannabinol (∆8-THC) | – | ND | – | ND | – | ND | – | ND |
| Cannabicyclol (CBL) | – | ND | – | ND | – | ND | – | NA |
| Cannabichromene (CBC) | 1.34 | 0.13 | 0.61 | 0.06 | 0.43 | 0.04 | – | ND |
| Tetrahydrocannabinolic acid A (THCA-A) | 0.68 | 0.07 | 0.31 | 0.03 | – | ND | – | ND |
| Cannabichromenic acid (CBCA) | 1.64 | 0.16 | 0.73 | 0.07 | 0.58 | 0.06 | – | ND |
| Unique Cannabinoids Detected: | 9 | – | 7 | – | 6 | – | 3 | – |
| Total Cannabinoid Content: | 243.00 | 24.30 | 89.29 | 8.93 | 60.32 | 6.03 | 120.30 | 12.03 |
| Total THC Content: | 1.37 | 0.14 | 0.62 | 0.06 | 0.27 | 0.03 | 0.00 | ND |
| Total CBD Content: | 16.58 | 1.66 | 0.00 | 0.00 | 0.00 | 0.00 | 0.80 | 0.08 |
| Analyte | Method 4 | Method 5 | Method 6 | C of A | ||||
| mg/g | wt % | mg/g | wt % | mg/g | wt % | mg/g | wt % | |
| Cannabidivarinic acid (CBDVA) | – | ND | – | ND | – | ND | – | ND |
| Cannabidivarin (CBDV) | 0.18 | 0.02 | – | ND | 0.52 | 0.05 | – | ND |
| Cannabidiolic acid (CBDA) | – | ND | – | ND | 0.10 | 0.01 | – | ND |
| Cannabigerolic acid (CBGA) | 82.23 | 8.22 | 59.68 | 5.97 | 64.69 | 6.47 | 113.50 | 11.35 |
| Cannabigerol (CBG) | 20.58 | 2.06 | 38.52 | 3.85 | 19.34 | 1.93 | 6.00 | 0.60 |
| Cannabidiol (CBD) | – | ND | – | ND | 0.15 | 0.01 | 0.80 | 0.08 |
| Tetrahydrocannabivarin (THCV) | 0.18 | 0.02 | – | ND | 0.16 | 0.02 | – | ND |
| Tetrahydrocannabivarinic acid (THCVA) | 0.12 | 0.01 | – | ND | 0.08 | 0.01 | – | ND |
| Cannabinol (CBN) | – | ND | – | ND | – | ND | – | ND |
| Cannabinolic acid (CBNA) | – | ND | – | ND | 0.10 | 0.01 | – | ND |
| Delta 9 Tetrahydrocannabinol (∆9-THC) | 0.35 | 0.04 | – | ND | 0.44 | 0.04 | – | ND |
| Delta 8 Tetrahydrocannabinol (∆8-THC) | – | ND | – | ND | – | ND | – | ND |
| Cannabicyclol (CBL) | – | ND | – | ND | – | ND | – | NA |
| Cannabichromene (CBC) | 0.64 | 0.06 | – | ND | 0.62 | 0.06 | – | ND |
| Tetrahydrocannabinolic acid A (THCA-A) | 0.35 | 0.04 | – | ND | 0.10 | 0.01 | – | ND |
| Cannabichromenic acid (CBCA) | 0.74 | 0.07 | – | ND | 1.04 | 0.10 | – | ND |
| Unique Cannabinoids Detected: | 9 | – | 2 | – | 12 | – | 3 | – |
| Total Cannabinoid Content: | 105.39 | 10.54 | 98.21 | 9.82 | 87.34 | 8.73 | 120.30 | 12.03 |
| Total THC Content: | 0.66 | 0.07 | 0.00 | 0.00 | 0.52 | 0.05 | 0.00 | ND |
| Total CBD Content: | 0.00 | 0.00 | 0.00 | 0.00 | 0.24 | 0.02 | 0.80 | 0.08 |
Table V: Calculated cannabinoid content in second extracts using chemovar #1 (CBG dominant).
| Analyte | Method 1 | Method 2 | Method 3 | |||
| mg/g | wt % | mg/g | wt % | mg/g | wt % | |
| Cannabidivarinic acid (CBDVA) | ND | – | ND | – | ND | – |
| Cannabidivarin (CBDV) | ND | – | ND | – | ND | – |
| Cannabidiolic acid (CBDA) | ND | – | ND | – | ND | – |
| Cannabigerolic acid (CBGA) | 0.92 | 0.09 | ND | – | 0.11 | 0.01 |
| Cannabigerol (CBG) | 0.38 | 0.04 | 0.16 | 0.02 | 0.17 | 0.02 |
| Cannabidiol (CBD) | – | ND | – | ND | – | ND |
| Tetrahydrocannabivarin (THCV) | – | ND | – | ND | – | ND |
| Tetrahydrocannabivarinic acid (THCVA) | – | ND | – | ND | – | ND |
| Cannabinol (CBN) | – | ND | – | ND | – | ND |
| Cannabinolic acid (CBNA) | – | ND | – | ND | – | ND |
| Delta 9 Tetrahydrocannabinol (∆9-THC) | – | ND | – | ND | – | ND |
| Delta 8 Tetrahydrocannabinol (∆8-THC) | – | ND | – | ND | – | ND |
| Cannabicyclol (CBL) | – | ND | – | ND | – | ND |
| Cannabichromene (CBC) | – | ND | – | ND | – | ND |
| Tetrahydrocannabinolic acid A (THCA-A) | – | ND | – | ND | – | ND |
| Cannabichromenic acid (CBCA) | – | ND | – | ND | – | ND |
| Total Cannabinoid Content: | 1.31 | 0.13 | 0.16 | 0.02 | 0.28 | 0.03 |
| Analyte | Method 4 | Method 5 | Method 6 | |||
| mg/g | wt % | mg/g | wt % | mg/g | wt % | |
| Cannabidivarinic acid (CBDVA) | ND | – | ND | – | ND | – |
| Cannabidivarin (CBDV) | ND | – | ND | – | ND | – |
| Cannabidiolic acid (CBDA) | ND | – | ND | – | ND | – |
| Cannabigerolic acid (CBGA) | 0.37 | 0.04 | ND | – | 1.52 | 0.15 |
| Cannabigerol (CBG) | 0.31 | 0.03 | 0.31 | 0.03 | 0.57 | 0.06 |
| Cannabidiol (CBD) | ND | – | ND | – | ND | – |
| Tetrahydrocannabivarin (THCV) | ND | – | ND | – | ND | – |
| Tetrahydrocannabivarinic acid (THCVA) | ND | – | ND | – | ND | – |
| Cannabinol (CBN) | ND | – | ND | – | ND | – |
| Cannabinolic acid (CBNA) | ND | – | ND | – | ND | – |
| Delta 9 Tetrahydrocannabinol (∆9-THC) | ND | – | ND | – | ND | – |
| Delta 8 Tetrahydrocannabinol (∆8-THC) | ND | – | ND | – | ND | – |
| Cannabicyclol (CBL) | ND | – | ND | – | ND | – |
| Cannabichromene (CBC) | ND | – | ND | – | ND | – |
| Tetrahydrocannabinolic acid A (THCA-A) | 0.19 | 0.02 | ND | – | ND | – |
| Cannabichromenic acid (CBCA) | 0.33 | 0.03 | ND | – | ND | – |
| Total Cannabinoid Content: | 1.20 | 0.12 | 0.31 | 0.03 | 2.09 | 0.21 |
Table VI: Calculated cannabinoid content in first extracts using chemovar #2 (CBD dominant).
| Analyte | Method 1 | Method 2 | Method 3 | C of A | ||||
| mg/g | wt % | mg/g | wt % | mg/g | wt % | mg/g | wt % | |
| Cannabidivarinic acid (CBDVA) | 0.55 | 0.06 | 0.11 | 0.01 | – | ND | 0.90 | 0.09 |
| Cannabidivarin (CBDV) | 1.61 | 0.16 | 0.68 | 0.07 | 0.54 | 0.05 | – | ND |
| Cannabidiolic acid (CBDA) | 177.05 | 17.70 | 146.37 | 14.64 | 129.75 | 12.97 | 195.00 | 19.5 |
| Cannabigerolic acid (CBGA) | 7.73 | 0.77 | 2.24 | 0.22 | 2.88 | 0.29 | 5.00 | 0.50 |
| Cannabigerol (CBG) | 2.66 | 0.27 | 1.77 | 0.18 | 2.20 | 0.22 | 0.60 | 0.06 |
| Cannabidiol (CBD) | 45.95 | 4.60 | 21.77 | 2.18 | 20.31 | 2.03 | 3.20 | 0.32 |
| Tetrahydrocannabivarin (THCV) | 1.47 | 0.15 | 2.70 | 0.27 | – | ND | – | ND |
| Tetrahydrocannabivarinic acid (THCVA) | – | ND | – | ND | – | ND | – | ND |
| Cannabinol (CBN) | – | ND | – | ND | – | ND | – | ND |
| Cannabinolic acid (CBNA) | 0.58 | 0.06 | 2.29 | 0.23 | 0.32 | 0.03 | – | ND |
| Delta 9 Tetrahydrocannabinol (∆9-THC) | 7.33 | 0.73 | 4.79 | 0.48 | 4.33 | 0.43 | 1.70 | 0.17 |
| Delta 8 Tetrahydrocannabinol (∆8-THC) | – | ND | – | ND | – | ND | ND | ND |
| Cannabicyclol (CBL) | 2.33 | 0.23 | 0.88 | 0.09 | 0.32 | 0.03 | NA | NA |
| Cannabichromene (CBC) | 5.85 | 0.58 | 3.47 | 0.35 | 3.06 | 0.31 | 0.20 | 0.02 |
| Tetrahydrocannabinolic acid A (THCA-A) | 6.25 | 0.63 | 3.94 | 0.39 | 3.80 | 0.38 | 7.60 | 0.76 |
| Cannabichromenic acid (CBCA) | 16.97 | 1.70 | 8.14 | 0.81 | 8.67 | 0.87 | 11.50 | 1.15 |
| Unique Cannabinoids Detected: | 13 | – | 13 | – | 11 | – | 9 | – |
| Total Cannabinoid Content: | 276.88 | 27.69 | 199.13 | 19.91 | 176.18 | 17.62 | 225.70 | 22.57 |
| Total THC Content: | 12.81 | 1.28 | 8.24 | 0.82 | 7.66 | 0.77 | 8.37 | 0.84 |
| Total CBD Content: | 201.22 | 20.12 | 150.14 | 15.01 | 134.10 | 13.41 | 174.22 | 17.42 |
| Analyte | Method 4 | Method 5 | Method 6 | C of A | ||||
| mg/g | wt % | mg/g | wt % | mg/g | wt % | mg/g | wt % | |
| Cannabidivarinic acid (CBDVA) | 0.76 | 0.08 | – | ND | 1.12 | 0.11 | 0.90 | 0.09 |
| Cannabidivarin (CBDV) | 1.10 | 0.11 | 0.88 | 0.09 | 1.24 | 0.12 | – | ND |
| Cannabidiolic acid (CBDA) | 188.10 | 18.81 | 160.10 | 16.01 | 151.06 | 15.11 | 195.00 | 19.50 |
| Cannabigerolic acid (CBGA) | 3.01 | 0.30 | 3.16 | 0.32 | 4.18 | 0.42 | 5.00 | 0.50 |
| Cannabigerol (CBG) | 2.73 | 0.27 | 1.77 | 0.18 | 2.40 | 0.24 | 0.60 | 0.06 |
| Cannabidiol (CBD) | 28.60 | 2.86 | 34.51 | 3.45 | 25.77 | 2.58 | 3.20 | 0.32 |
| Tetrahydrocannabivarin (THCV) | 3.67 | 0.37 | – | ND | 0.66 | 0.07 | – | ND |
| Tetrahydrocannabivarinic acid (THCVA) | – | ND | – | ND | – | ND | – | ND |
| Cannabinol (CBN) | – | ND | – | ND | – | ND | – | ND |
| Cannabinolic acid (CBNA) | 0.36 | 0.04 | – | ND | 0.16 | 0.02 | – | ND |
| Delta 9 Tetrahydrocannabinol (∆9-THC) | 5.91 | 0.59 | 7.54 | 0.75 | 7.06 | 0.71 | 1.70 | 0.17 |
| Delta 8 Tetrahydrocannabinol (∆8-THC) | – | ND | – | ND | – | ND | ND | ND |
| Cannabicyclol (CBL) | 1.20 | 0.12 | 1.56 | 0.16 | 1.36 | 0.14 | NA | NA |
| Cannabichromene (CBC) | 3.87 | 0.39 | 5.98 | 0.60 | 4.50 | 0.45 | 0.20 | 0.02 |
| Tetrahydrocannabinolic acid A (THCA-A) | 5.23 | 0.52 | 3.24 | 0.32 | 5.74 | 0.57 | 7.60 | 0.76 |
| Cannabichromenic acid (CBCA) | 16.65 | 1.67 | 7.52 | 0.75 | 10.80 | 1.08 | 11.50 | 1.15 |
| Unique Cannabinoids Detected: | 13 | – | 10 | – | 13 | – | 9 | – |
| Total Cannabinoid Content: | 261.19 | 26.12 | 226.25 | 22.63 | 216.07 | 21.61 | 225.70 | 22.57 |
| Total THC Content: | 10.49 | 1.05 | 10.38 | 1.04 | 12.10 | 1.21 | 8.37 | 0.84 |
| Total CBD Content: | 193.57 | 19.36 | 174.92 | 17.49 | 158.25 | 15.83 | 174.22 | 17.42 |
Table VII: Calculated cannabinoid content in second extracts using chemovar #2 (CBD dominant).
| Analyte | Method 1 | Method 2 | Method 3 | |||
| mg/g | wt % | mg/g | wt % | mg/g | wt % | |
| Cannabidivarinic acid (CBDVA) | – | ND | – | ND | – | ND |
| Cannabidivarin (CBDV) | – | ND | – | ND | – | ND |
| Cannabidiolic acid (CBDA) | 2.58 | 0.22 | – | ND | – | ND |
| Cannabigerolic acid (CBGA) | – | ND | – | ND | – | ND |
| Cannabigerol (CBG) | 0.49 | 0.05 | – | ND | – | ND |
| Cannabidiol (CBD) | 0.43 | 0.04 | 0.43 | 0.04 | 0.38 | 0.04 |
| Tetrahydrocannabivarin (THCV) | – | ND | – | ND | – | ND |
| Tetrahydrocannabivarinic acid (THCVA) | – | ND | – | ND | – | ND |
| Cannabinol (CBN) | – | ND | – | ND | – | ND |
| Cannabinolic acid (CBNA) | – | ND | – | ND | – | ND |
| Delta 9 Tetrahydrocannabinol (∆9-THC) | 0.31 | 0.03 | – | ND | – | ND |
| Delta 8 Tetrahydrocannabinol (∆8-THC) | – | ND | – | ND | – | ND |
| Cannabicyclol (CBL) | – | ND | – | ND | – | ND |
| Cannabichromene (CBC) | 0.17 | 0.02 | – | ND | – | ND |
| Tetrahydrocannabinolic acid A (THCA-A) | 0.22 | 0.02 | – | ND | – | ND |
| Cannabichromenic acid (CBCA) | 0.37 | 0.04 | – | ND | – | ND |
| Total Cannabinoid Content: | 4.15 | 0.42 | 0.43 | 0.04 | 0.38 | 0.04 |
| Analyte | Method 4 | Method 5 | Method 6 | |||
| mg/g | wt % | mg/g | wt % | mg/g | wt % | |
| Cannabidivarinic acid (CBDVA) | – | ND | – | ND | – | ND |
| Cannabidivarin (CBDV) | – | ND | – | ND | – | ND |
| Cannabidiolic acid (CBDA) | 1.14 | 0.11 | 0.68 | 0.07 | 4.14 | 0.41 |
| Cannabigerolic acid (CBGA) | – | ND | – | ND | – | ND |
| Cannabigerol (CBG) | – | ND | – | ND | – | ND |
| Cannabidiol (CBD) | 0.56 | 0.06 | 0.62 | 0.06 | 0.66 | 0.07 |
| Tetrahydrocannabivarin (THCV) | – | ND | – | ND | – | ND |
| Tetrahydrocannabivarinic acid (THCVA) | – | ND | – | ND | – | ND |
| Cannabinol (CBN) | – | ND | – | ND | – | ND |
| Cannabinolic acid (CBNA) | – | ND | – | ND | – | ND |
| Delta 9 Tetrahydrocannabinol (∆9-THC) | – | ND | – | ND | 0.13 | 0.01 |
| Delta 8 Tetrahydrocannabinol (∆8-THC) | – | ND | – | ND | – | ND |
| Cannabicyclol (CBL) | – | ND | – | ND | 0.07 | 0.01 |
| Cannabichromene (CBC) | 0.15 | 0.02 | – | ND | 0.09 | 0.01 |
| Tetrahydrocannabinolic acid A (THCA-A) | 0.20 | 0.02 | – | ND | 0.12 | 0.01 |
| Cannabichromenic acid (CBCA) | 0.35 | 0.03 | – | ND | 0.29 | 0.03 |
| Total Cannabinoid Content: | 2.39 | 0.24 | 1.30 | 0.13 | 5.49 | 0.55 |
Determining Extraction Efficiency
Once cannabinoid content was determined, extraction efficiency was calculated using formula 5. Results are shown in Tables VIII and IX for the CBG-dominant and CBD-dominant chemovars, respectively.
Formula 5:

Where E1 and E2 are 1st total cannabinoid extraction and 2nd total cannabinoid extraction content respectively.
Table VIII: Cannabinoid extraction efficiency for CBG hemp flower calculated using formula 5.
| Chemovar 1 – CBG | Total Cannabinoid Extracted | ||
| Cannabinoids Recovered 1st Extraction (mg/g) | Cannabinoids Recovered 2nd Extraction (mg/g) | Extraction Efficiency (%) | |
| Method 1 | 243.00 | 1.31 | 99.46 |
| Method 2 | 89.29 | 0.16 | 99.83 |
| Method 3 | 60.32 | 0.28 | 99.54 |
| Method 4 | 105.39 | 1.20 | 98.86 |
| Method 5 | 98.21 | 0.31 | 99.68 |
| Method 6 | 87.34 | 2.09 | 97.61 |
Table IX: Cannabinoid extraction efficiency for CBD hemp flower using formula 5.
| Chemovar 2 – CBD | Total Cannabinoid Extracted | ||
| Cannabinoids Recovered 1st Extraction (mg/g) | Cannabinoids Recovered 2nd Extraction (mg/g) | Extraction Efficiency (%) | |
| Method 1 | 276.88 | 4.15 | 98.50 |
| Method 2 | 199.13 | 0.43 | 99.77 |
| Method 3 | 176.18 | 0.38 | 99.79 |
| Method 4 | 261.19 | 2.39 | 99.08 |
| Method 5 | 226.25 | 1.30 | 99.43 |
| Method 6 | 216.07 | 5.49 | 97.46 |
To ensure cannabinoid extraction efficiency is similar across different varieties, the extraction efficiencies were compared, and percent difference was calculated for each extraction method using formula 6. The results in Table X suggests these methods are transferable across different varieties.
Formula 6:
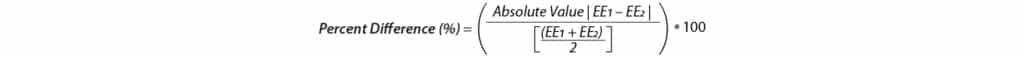
Where EE1 and EE2 are Extraction Efficiency of chemovar 1 and Extraction Efficiency of chemovar 2, respectively.
Table X: Comparison of cannabinoid extraction efficiencies between two chemovars.
| Sample Prep Method | Chemovar 1 | Chemovar 2 | Percent Difference (%) |
| Extraction Efficiency (%) | Extraction Efficiency (%) | ||
| Method 1 | 99.46 | 98.50 | 0.97 |
| Method 2 | 99.83 | 99.77 | 0.06 |
| Method 3 | 99.54 | 99.79 | 0.25 |
| Method 4 | 98.86 | 99.08 | 0.22 |
| Method 5 | 99.68 | 99.43 | 0.25 |
| Method 6 | 97.61 | 97.46 | 0.15 |
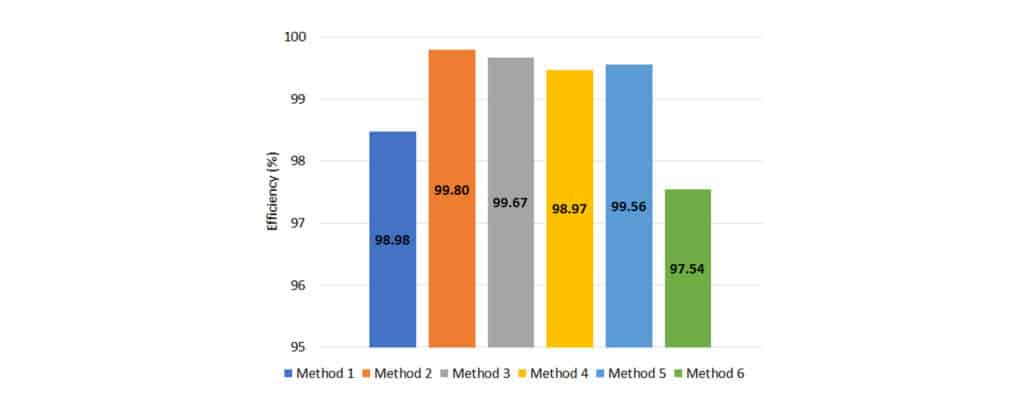
To determine overall extraction efficiency for each sample preparation method, the results for chemovar 1 and 2 were averaged. The average cannabinoid extraction efficiencies for each procedure can be seen in Figure 3.
Figure 3: Average extraction efficiency across both chemovars.
Sample Preparation Time
When determining the best overall method, it is also important to consider sample preparation time. Often, to improve productivity and profitability, labs search for a rapid procedure to ensure high sample throughput capacity. Figure 4 shows a comparison of sample preparation time for each extraction method.
Figure 4: Sample preparation time for each cannabinoid extraction method.
Direct Comparison
Evaluation of the extraction methods has demonstrated that many are either very costly, require a significant amount of sample preparation time, or differ in the individual cannabinoids and concentrations that can be determined. In fact, some of the procedures do not offer an acceptable return on investment for production labs that are very mindful of cost per sample. For a final experiment, the extraction methods that used a large amount of solvent, were relatively expensive, or had a long sample preparation time were eliminated. The three that did meet acceptability criteria were Method 1, Method 4, and Method 6. Method 5 was also included in a final comparison because it is a relatively new procedure and is still out for public comment.
In the initial comparisons, different quantities of sample matrix were specified (100–500 mg) in each procedure. To provide a direct comparison, the same sample size (500 mg) of a CBD-dominant chemovar was used. While sample size was increased, the remainder of the prescribed methods stayed the same because scaling the solvent volume with the sample mass would significantly increase the cost of sample preparation. The cannabinoid content for both extractions of all four remaining methods and the certified values for the chemovar are presented in Tables XI and XII.
Table XI: Calculated cannabinoid content in the first extraction of 500 mg of chemovar #2.
| Analyte | Method 1 | Method 4 | C of A | |||
| mg/g | wt % | mg/g | wt % | mg/g | wt % | |
| Cannabidivarinic acid (CBDVA) | 0.67 | 0.07 | 0.54 | 0.05 | 0.9 | 0.09 |
| Cannabidivarin (CBDV) | 1.32 | 0.13 | 1.30 | 0.13 | – | ND |
| Cannabidiolic acid (CBDA) | 147.01 | 14.7 | 169.18 | 16.92 | 195.00 | 19.5 |
| Cannabigerolic acid (CBGA) | 3.80 | 0.38 | 4.00 | 0.40 | 5.00 | 0.5 |
| Cannabigerol (CBG) | 1.70 | 0.17 | 2.84 | 0.28 | 0.60 | 0.06 |
| Cannabidiol (CBD) | 34.00 | 3.4 | 36.87 | 3.69 | 3.20 | 0.32 |
| Tetrahydrocannabivarin (THCV) | 0.22 | 0.02 | 0.50 | 0.05 | – | ND |
| Tetrahydrocannabivarinic acid (THCVA) | 0.14 | 0.01 | 0.17 | 0.02 | – | ND |
| Cannabinol (CBN) | – | ND | – | ND | – | ND |
| Cannabinolic acid (CBNA) | 1.78 | 0.18 | 1.81 | 0.18 | – | ND |
| Delta 9 Tetrahydrocannabinol (∆9-THC) | 12.10 | 1.21 | 8.15 | 0.81 | 1.70 | 0.17 |
| Delta 8 Tetrahydrocannabinol (∆8-THC) | – | ND | – | ND | ND | ND |
| Cannabicyclol (CBL) | 1.4 | 0.14 | 1.48 | 0.15 | NA | NA |
| Cannabichromene (CBC) | 8.15 | 0.82 | 6.14 | 0.61 | 0.2 | 0.02 |
| Tetrahydrocannabinolic acid A (THCA-A) | 2.02 | 0.2 | 3.32 | 0.33 | 7.6 | 0.76 |
| Cannabichromenic acid (CBCA) | 5.79 | 0.58 | 8.46 | 0.85 | 11.5 | 1.15 |
| Unique Cannabinoids Detected: | 14 | – | 14 | – | 9 | – |
| Total Cannabinoid Content: | 220.1 | 22.01 | 244.77 | 24.48 | 225.7 | 22.57 |
| Total THC Content: | 13.87 | 1.39 | 11.06 | 1.11 | 8.37 | 0.80 |
| Total CBD Content: | 162.93 | 16.29 | 185.24 | 18.52 | 174.22 | 17.42 |
| Analyte | Method 5 | Method 6 | C of A | |||
| mg/g | wt % | mg/g | wt % | mg/g | wt % | |
| Cannabidivarinic acid (CBDVA) | 0.21 | 0.02 | 1.12 | 0.11 | 0.9 | 0.09 |
| Cannabidivarin (CBDV) | 1.23 | 0.12 | 1.24 | 0.12 | – | ND |
| Cannabidiolic acid (CBDA) | 162.88 | 16.29 | 151.06 | 15.11 | 195.00 | 19.5 |
| Cannabigerolic acid (CBGA) | 3.89 | 0.39 | 4.18 | 0.42 | 5.00 | 0.5 |
| Cannabigerol (CBG) | 2.16 | 0.22 | 2.40 | 0.24 | 0.60 | 0.06 |
| Cannabidiol (CBD) | 33.56 | 3.36 | 25.77 | 2.58 | 3.20 | 0.32 |
| Tetrahydrocannabivarin (THCV) | 1.44 | 0.14 | 0.66 | 0.07 | – | ND |
| Tetrahydrocannabivarinic acid (THCVA) | – | ND | – | ND | – | ND |
| Cannabinol (CBN) | – | ND | – | ND | – | ND |
| Cannabinolic acid (CBNA) | – | ND | 0.16 | 0.02 | – | ND |
| Delta 9 Tetrahydrocannabinol (∆9-THC) | 5.77 | 0.58 | 7.06 | 0.71 | 1.70 | 0.17 |
| Delta 8 Tetrahydrocannabinol (∆8-THC) | – | ND | – | ND | ND | ND |
| Cannabicyclol (CBL) | 1.23 | 0.12 | 1.36 | 0.14 | NA | NA |
| Cannabichromene (CBC) | 4.44 | 0.44 | 4.50 | 0.45 | 0.2 | 0.02 |
| Tetrahydrocannabinolic acid A (THCA-A) | 2.43 | 0.24 | 5.74 | 0.57 | 7.6 | 0.76 |
| Cannabichromenic acid (CBCA) | 6.24 | 0.62 | 10.80 | 1.08 | 11.5 | 1.15 |
| Unique Cannabinoids Detected: | 14 | – | 13 | – | 9 | – |
| Total Cannabinoid Content: | 225.46 | 22.55 | 216.07 | 21.61 | 225.7 | 22.57 |
| Total THC Content: | 7.90 | 0.79 | 12.10 | 1.21 | 8.37 | 0.80 |
| Total CBD Content: | 176.40 | 17.64 | 158.25 | 15.83 | 174.22 | 17.42 |
Table XII: Calculated cannabinoid content in the second extraction of 500 mg of chemovar #2.
| Analyte | Method 1 | Method 4 | Method 5 | Method 6 | ||||
| mg/g | wt % | mg/g | wt % | mg/g | wt % | mg/g | wt % | |
| Cannabidivarinic acid (CBDVA) | – | ND | – | ND | – | ND | – | ND |
| Cannabidivarin (CBDV) | – | ND | – | ND | – | ND | – | ND |
| Cannabidiolic acid (CBDA) | 6.14 | 0.61 | 7.56 | 0.76 | 1.95 | 0.19 | 4.14 | 0.41 |
| Cannabigerolic acid (CBGA) | – | ND | 0.18 | 0.02 | – | ND | – | ND |
| Cannabigerol (CBG) | 0.08 | 0.01 | 0.09 | 0.01 | 0.61 | 0.06 | – | ND |
| Cannabidiol (CBD) | 1.63 | 0.16 | 2.07 | 0.21 | – | ND | 0.66 | 0.07 |
| Tetrahydrocannabivarin (THCV) | – | ND | – | ND | – | ND | – | ND |
| Tetrahydrocannabivarinic acid (THCVA) | – | ND | – | ND | – | ND | – | ND |
| Cannabinol (CBN) | – | ND | – | ND | – | ND | – | ND |
| Cannabinolic acid (CBNA) | – | ND | – | ND | – | ND | – | ND |
| Delta 9 Tetrahydrocannabinol (∆9-THC) | 0.28 | 0.03 | 0.38 | 0.04 | – | ND | 0.13 | 0.01 |
| Delta 8 Tetrahydrocannabinol (∆8-THC) | – | ND | – | ND | – | ND | – | ND |
| Cannabicyclol (CBL) | 0.21 | 0.02 | 0.12 | 0.01 | – | ND | 0.07 | 0.01 |
| Cannabichromene (CBC) | 0.23 | 0.02 | 0.33 | 0.03 | – | ND | 0.09 | 0.01 |
| Tetrahydrocannabinolic acid A (THCA-A) | 0.14 | 0.01 | 0.22 | 0.02 | – | ND | 0.12 | 0.01 |
| Cannabichromenic acid (CBCA) | 0.37 | 0.04 | 0.63 | 0.06 | – | ND | 0.29 | 0.03 |
| Total Cannabinoid Content: | 9.09 | 0.91 | 11.60 | 1.16 | 2.56 | 0.26 | 5.49 | 0.55 |
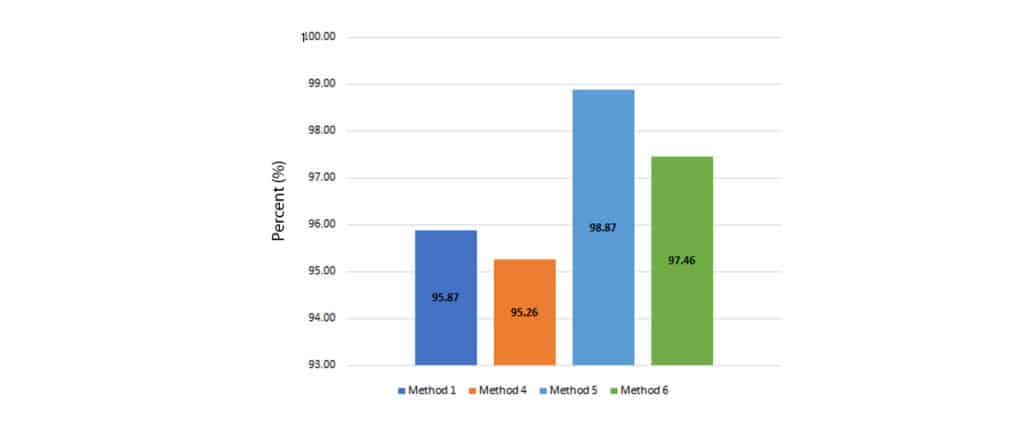
Using formula 5, cannabinoid extraction efficiency was calculated, and results can be seen in Figure 5. All methods were >95% efficient using 500 mg.
Figure 5: Cannabinoid extraction efficiency of four sample preparation methods using 500 mg of sample.
The effect of sample size was also examined by comparing data for the original sample size (100 or 200 mg) to the 500 mg samples. Results are presented in Table XIII, and the comparison suggests that, for these methods, extraction efficiency decreases as sample size increases. Note that for Method 6 a 200 mg sample was also included because a 500 mg sample was used in the original experiment.
Table XIII: Comparison results of prescribed method weight vs. 500 mg weight.
| Method 1 | Method 4 | Method 5 | Method 6 | |||||
| 200 mg | 500 mg | 100 mg | 500 mg | 200 mg | 500 mg | 200 mg | 500 mg | |
| Unique Cannabinoids Detected: | 13 | 14 | 13 | 14 | 10 | 14 | 13 | 13 |
| Total Cannabinoid Content: (wt %) | 27.69 | 22.01 | 26.12 | 24.48 | 22.63 | 22.55 | 21.61 | 21.61 |
| Total THC Content: (wt %) | 1.28 | 1.39 | 1.05 | 1.11 | 1.04 | 0.79 | 1.21 | 1.21 |
| Total CBD Content: (wt %) | 20.12 | 16.29 | 19.36 | 18.52 | 17.49 | 17.64 | 15.83 | 15.83 |
| Extraction Efficiency: (%) | 98.50 | 95.87 | 99.08 | 95.26 | 99.43 | 98.87 | 97.61 | 97.46 |
| Percent Difference: (%) | 2.71 | 3.93 | 0.56 | 0.15 | ||||
Final Overview
The four extraction methods evaluated in the latter part of this study can be broken down by solvent cost, extraction efficiency, consumables cost, and sample preparation time. Each procedure was given a score in each category from 1 to 4 with 4 being the best (Table XIV). For each extraction method, scores from each category were added together, and each procedure was given an overall rating (Table XV).
Table XIV: Scores for each category for the top four extraction methods.
| Category | Extraction Method | Result | Score |
| Extraction Solvent Cost/Sample ($) | Method 1 | $0.77 | 2 |
| Method 4 | $0.42 | 3 | |
| Method 5 | $3.76 | 1 | |
| Method 6 | $0.38 | 4 | |
| Extraction Efficiency (%) @ 500 mg | Method 1 | 95.87% | 2 |
| Method 4 | 95.26% | 1 | |
| Method 5 | 98.87% | 4 | |
| Method 6 | 97.46% | 3 | |
| Sample Prep Time (min) | Method 1 | 20 | 3 |
| Method 4 | 18 | 4 | |
| Method 5 | 51 | 1 | |
| Method 6 | 31.5 | 2 | |
| Consumables Cost/Sample ($) | Method 1 | $3.29 | 4 |
| Method 4 | $4.20 | 3 | |
| Method 5 | $4.63 | 2 | |
| Method 6 | $5.09 | 1 |
Table XV: Final scores and overall ratings for four methods.
| Extraction Method | Total Score | Rating |
| Method 1 | 11 | 1 |
| Method 4 | 11 | 1 |
| Method 5 | 8 | 3 |
| Method 6 | 10 | 2 |
While Methods 1 and 4 had the highest overall ranking, the selection of a sample preparation procedure ultimately boils down to the priorities and requirements of each lab, and there were clear differences among the cannabinoid extraction methods tested in certain performance areas. Some labs may want to sacrifice the detection of unique cannabinoids for solvent cost savings, while others may prefer a shorter sample preparation time. To determine which sample extraction method is truly best, labs should identify their priorities (regulatory, cost, equipment, compounds, etc.) and choose the method that best meets those specific needs. Note also that this research focused solely on sample preparation; when developing a full potency method, labs also need to consider that some governing entities may require specific instrument parameters that were outside the scope of this study.
References
- Storm, M. Zumwalt, A. Macherone, Dedicated cannabinoid potency testing in cannabis or hemp products using the Agilent 1220 Infinity II LC system, Application note, Agilent, 2020. https://www.agilent.com/cs/library/applications/application-dedicated-cannabinoid-potency-testing-5991-9285-en-us-agilent.pdf (accessed June 2022).
- Analysis of 17 cannabinoids in hemp and cannabis, MilliporeSigma. https://www.sigmaaldrich.com/US/en/technical-documents/protocol/analytical-chemistry/small-molecule-hplc/analysis-of-cannabinoids (accessed June 2022).
- L. Vaclavik, F. Benes, M. Fenclova, J. Hricko, A. Krmela, V. Svobodova, J. Hajslova, K. Mastovska, Quantitation of cannabinoids in cannabis dried plant materials, concentrates, and oils using liquid chromatography–diode array detection technique with optional mass spectrometric detection: single-laboratory validation study, first action 2018.11, J. AOAC Int., 102 (6) (2019) 1822–1833. https://pubmed.ncbi.nlm.nih.gov/31208494/
- ASTM, Method WK67498, New test method for determination of cannabinoid concentration in cannabis using liquid chromatography tandem mass spectrometry (LC-MS/MS). https://www.astm.org/workitem-wk67498 (accessed January 2022).
- Department of Cannabis Control, Chapter 6 Testing Laboratories, Article 4 Standard Operating Procedures, 15712.1 Test Methods for Cannabinoids, 2022. https://cannabis.ca.gov/wp-content/uploads/sites/2/2022/08/Cannabinoids_Test_Method_15-Day_Modified_Text_of_Regulations_and_SOP_8.25.2022.pdf (accessed June 2022).


