Abstract
In this study, a simplified sample preparation procedure and a reliable LC-MS/MS analytical method were developed for the simultaneous analysis of 37 regulated and emerging mycotoxins, including five Alternaria toxins, six major ergot alkaloids and their corresponding epimers, and priority mycotoxins from other classes. Four different food matrices (wheat baby cereal, peanut, tomato puree, and blended flour) were evaluated to demonstrate the applicability of this method to a wide range of food types.
Introduction
Mycotoxins, which are produced by a diverse range of fungi, are widespread food contaminants that have significant impacts on both human health and global economics. The prevalence and co-occurrence of different classes of mycotoxins vary greatly among geographic location and crop types, which has resulted in a complex international landscape of regulations and maximum residue concentrations. A simple method that allows simultaneous determination of multiple mycotoxin classes in different food matrices would greatly improve test lab productivity, but comprehensive methods are difficult to develop due to the wide range of chemical properties exhibited by mycotoxins from different fungal species.
For multi-mycotoxin analysis, one of the most significant challenges is combining two emerging groups—Alternaria toxins and ergot alkaloids—into methods for other classes of mycotoxins. The most important Alternaria toxins are altenuene, alternariol, alternariol monomethylether, tentoxin, and tenuazonic acid (TeA), which are frequently detected in cereal- and fruit-based food products. Tenuazonic acid is typically present at much higher concentrations than other Alternaria toxins, and several studies have shown that high pH conditions are necessary to obtain acceptable peak shape for it when using a C18 column [1, 2]. Similarly, the most important ergot alkaloids (ergocornine, ergocristine, ergocryptine, ergometrine, ergosine, and ergotamine, together with their isomeric -inine epimers) require high pH LC conditions to achieve full separation on a C18 column [3, 4]. Use of high pH conditions is stressful for LC columns and not suitable for the chromatographic analysis of other classes of mycotoxins, so these conditions are not an ideal basis for comprehensive methods.
Another factor that complicates any comprehensive method is the need to mitigate matrix suppression or enhancement. Using cleanup procedures on sample extracts to reduce such matrix effects is common, but due to the variation in mycotoxin chemical characteristics, it is unlikely that a single cleanup protocol would produce proper recoveries and accurate quantification for all types of mycotoxins. The use of stable isotopes as internal standards is the most direct way to correct matrix effects, but, due to the lack of isotopically labeled compounds for most of mycotoxins, it is not feasible for comprehensive mycotoxin analysis [5]. Matrix-matched external standard calibration is a more suitable approach for multi-mycotoxin analysis, but it is dependent on finding a similar food matrix with relatively low levels of incurred mycotoxins to be used as the blank matrix.
In this study, we successfully developed a comprehensive quantitative method that is suitable for the simultaneous analysis of Alternaria toxins, ergot alkaloids and their epimers, and other major mycotoxins produced by Aspergillus, Fusarium, and Penicillium fungi. A simple sample preparation procedure was paired with fast, acidic LC conditions for accurate and robust analysis of 37 regulated and emerging mycotoxins. This method was developed on a Raptor Biphenyl column instead of a C18 column so desirable acidic conditions could be used. Four food matrices (wheat baby cereal, peanut, tomato puree, and blended flour) were used to demonstrate the applicability of this method to a wide range of food types. Matrix-matched calibration was used to offset matrix effects, and the method was demonstrated to be effective in terms of linearity, specificity, accuracy, precision, and adaptability.
Experimental
Sample Handling
Four different food matrices (wheat baby cereal, peanut, tomato puree, and blended flour) were chosen to demonstrate the applicability of this method to a wide range of food types. All sample commodities were purchased from local grocery stores. Wheat baby cereal and tomato puree were used in their original forms. Raw peanut was ground and stored in the refrigerator. A blended flour was prepared by mixing white rice flour (75%); brown rice flour (5%); millet flour (5%); oat flour (5%); all-purpose wheat flour (5%); and all-purpose, gluten-free flour (5%) with a handheld blender.
Sample and Matrix-Matched Standards Preparation
Two grams of sample were weighed into a 50-mL polypropylene centrifuge tube and fortified at 5, 50, and 200 µg/kg with stock standard solution. After sitting at room temperature for 10 minutes, 16 mL of 80:20 acetonitrile:water extraction solution containing 0.5% formic acid (no formic acid was used for tomato puree samples) were added and the tube was stirred to create a homogenous suspension. Extraction was carried out by shaking the samples horizontally on a digital pulse mixer (Glas-Col LLC, Terre Haute, IN) at 800 rpm for 20 minutes. After centrifuging for 5 minutes at 4000 rpm, 1 mL of extract was evaporated to dryness at 45 °C under a gentle stream of nitrogen. The dried extract was reconstituted with 1 mL of 50:50 water:methanol solution and a 0.4 mL aliquot was transferred to and filtered using a Thomson SINGLE StEP filter vial with a 0.2 µm PTFE filter (cat.# 25874).
Methanol was used instead of acetonitrile as the organic mobile phase because preliminary experiments determined that it generated better overall chromatographic performance in terms of proper retention and higher detection sensitivity for all analytes. While most multi-mycotoxin methods for food use aqueous acetonitrile for sample extraction, our testing showed that the early eluting compounds and ergot alkaloids did not form sharp, symmetrical peaks when an aqueous acetonitrile solution was injected. This could be corrected by using an aqueous methanol solution as the final diluent. Furthermore, certain mycotoxins, especially fumonisin B1, B2, and B3, had significantly reduced signal intensity in the aqueous acetonitrile solution. Based on these results, a 50:50 water:methanol diluent was used because it gave the best chromatographic performance for gradient elution.
To prepare matrix-matched calibration standards, the non-fortified matrices were extracted and dried down as described above. They were then reconstituted in a 50:50 water:methanol solution containing 0.05–50 ng/mL of the target mycotoxins, which is equivalent to a 0.4–400 µg/kg sample concentration range.
Chromatographic Method
Mycotoxin analysis was performed on a Waters ACQUITY I-class UPLC system coupled with a Xevo TQ-S triple quadrupole mass spectrometer. The chromatographic conditions were as follows, and the ion transitions used for each analyte are provided in Figure 1.
| Column: | Raptor Biphenyl, 2.7 µm, 100 mm x 2.1 mm (cat.# 9309A12) | |
| Guard column: | Raptor Biphenyl EXP guard column cartridge, 2.7 µm, 5 mm x 2.1 mm (cat.# 9309A0252) | |
| Mobile phase A: | Water, 0.05% formic acid | |
| Mobile phase B: | Methanol, 0.05% formic acid | |
| Gradient: | Time (min) | %B |
| 0.00 | 25 | |
| 5.00 | 50 | |
| 9.00 | 100 | |
| 9.01 | 25 | |
| 11.0 | 25 | |
| Flow rate: | 0.4 mL/min | |
| Injection volume: | 5 µL | |
| Column temp.: | 60 °C | |
| Ion mode: | Scheduled MRM, positive ESI | |
Results and Discussion
Chromatographic Performance
The primary reported drawback to mycotoxin analysis on C18 columns is that high pH conditions are required for Alternaria toxins and ergot alkaloids, which prohibits adding them to existing methods for other mycotoxins. Indeed, our preliminary experiments confirmed that when using a C18 column under acidic conditions, tenuazonic acid peak shape was too poor for integration and the ergot alkaloid epimers could not all be separated. In contrast, these emerging mycotoxins can be separated adequately and with satisfactory peak shapes under standard acidic conditions on a Raptor Biphenyl column, allowing for the development of a higher productivity combined method.
As shown in Figure 1, simultaneous analysis of 37 mycotoxins was achieved in a fast, 11-minute total cycle time run on a Raptor Biphenyl column under acidic conditions. Importantly, all epimer pairs of ergot alkaloids were chromatographically separated for definitive and accurate quantification. Epimer stability was evaluated during method development and no conversion was observed under the low pH conditions used here. In addition, quantifiable peak shape was obtained for tenuazonic acid, although method development work demonstrated that when a new column was installed it had to be rinsed and maintained under mobile phase overnight to ensure an acceptable peak shape for this challenging compound.
In addition to column selection, method development experiments also evaluated other parameters. Using a high column temperature (60 °C) was found to be critical for separating ergosine/ergosinine and ergotamine/ergotaminine epimers. Mobile phases containing various additives including formic acid, acetic acid, ammonium formate, and ammonium acetate were also tested. The best condition for both peak shape and sensitivity for most compounds was using formic acid as the single additive at 0.05% in both water (mobile phase A) and methanol (mobile phase B).
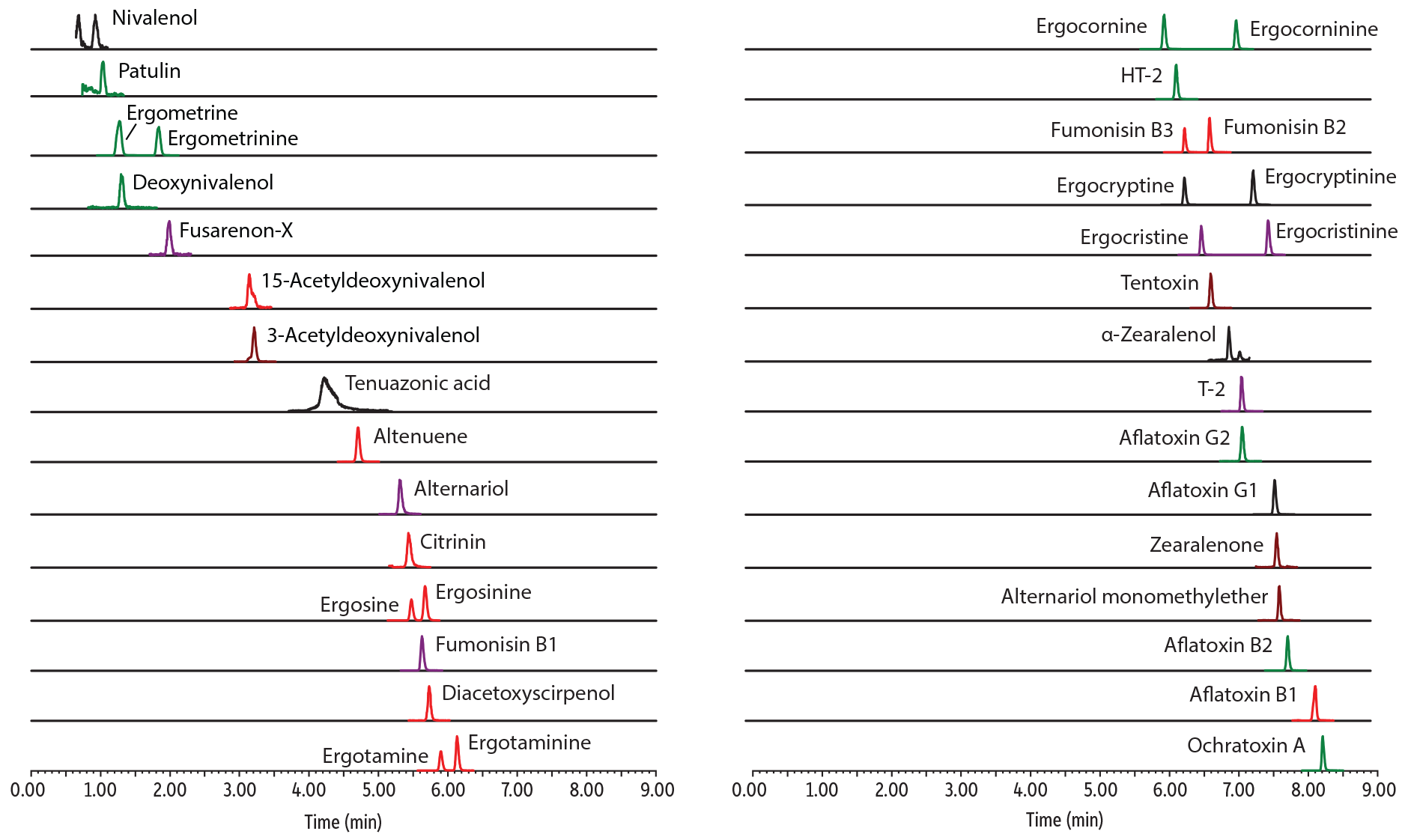
LC_FS0550
Peaks
| Peaks | tR (min) | Precursor Ion | Product Ion 1 | Product Ion 2 | |
|---|---|---|---|---|---|
| 1. | Nivalenol | 0.92 | 295.1 | 137.1 | 91.0 |
| 2. | Patulin | 1.03 | 155.0 | 99.0 | 81.0 |
| 3. | Ergometrine | 1.27 | 326.2 | 223.2 | 208.1 |
| 4. | Deoxynivalenol | 1.30 | 297.2 | 231.0 | 249.0 |
| 5. | Ergometrinine | 1.83 | 326.2 | 223.2 | 208.1 |
| 6. | Fusarenon-X | 1.98 | 355.1 | 137.1 | 247.1 |
| 7. | 15-Acetyldeoxynivalenol | 3.14 | 339.2 | 137.1 | 321.2 |
| 8. | 3-Acetyldeoxynivalenol | 3.21 | 339.2 | 213.1 | 231.1 |
| 9. | Tenuazonic acid | 4.22 | 198.1 | 125.0 | 153.1 |
| 10. | Altenuene | 4.70 | 293.2 | 257.1 | 275.2 |
| 11. | Alternariol | 5.30 | 259.0 | 185.1 | 130.0 |
| 12. | Citrinin | 5.43 | 251.2 | 233.1 | 205.1 |
| 13. | Ergosine | 5.47 | 548.4 | 208.1 | 223.2 |
| 14. | Fumonisin B1 | 5.63 | 722.5 | 352.3 | 334.2 |
| 15. | Ergosinine | 5.67 | 548.4 | 208.1 | 223.2 |
| 16. | Diacetoxyscirpenol | 5.73 | 384.2 | 247.1 | 307.2 |
| 17. | Ergotamine | 5.90 | 582.4 | 223.2 | 268.2 |
| 18. | Ergocornine | 6.03 | 562.4 | 268.2 | 223.2 |
| Peaks | tR (min) | Precursor Ion | Product Ion 1 | Product Ion 2 | |
|---|---|---|---|---|---|
| 19. | Ergotaminine | 6.13 | 582.4 | 223.2 | 268.2 |
| 20. | HT-2 | 6.20 | 447.2 | 345.1 | 285.1 |
| 21. | Fumonisin B3 | 6.32 | 706.4 | 336.2 | 318.3 |
| 22. | Ergocryptine | 6.32 | 576.4 | 268.2 | 223.2 |
| 23. | Ergocristine | 6.56 | 610.4 | 223.2 | 592.4 |
| 24. | Fumonisin B2 | 6.68 | 706.4 | 336.2 | 318.3 |
| 25. | Tentoxin | 6.70 | 415.2 | 312.2 | 302.2 |
| 26. | α-Zearalenol | 6.96 | 303.1 | 285.1 | 175.0 |
| 27. | Ergocorninine | 7.07 | 562.4 | 268.2 | 223.2 |
| 28. | T-2 | 7.14 | 489.2 | 387.1 | 245.1 |
| 29. | Aflatoxin G2 | 7.15 | 331.2 | 189.0 | 313.0 |
| 30. | Ergocryptinine | 7.31 | 576.4 | 268.2 | 223.2 |
| 31. | Ergocristinine | 7.53 | 610.4 | 223.2 | 592.4 |
| 32. | Aflatoxin G1 | 7.62 | 329.1 | 199.7 | 243.0 |
| 33. | Zearalenone | 7.65 | 319.2 | 283.1 | 187.0 |
| 34. | Alternariol monomethylether | 7.69 | 273.0 | 199.1 | 128.0 |
| 35. | Aflatoxin B2 | 7.81 | 315.1 | 287.0 | 259.0 |
| 36. | Aflatoxin B1 | 8.20 | 313.2 | 241.1 | 284.9 |
| 37. | Ochratoxin A | 8.31 | 404.1 | 239.0 | 358.0 |
Conditions
| Column | Raptor Biphenyl (cat.# 9309A12) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||
| Guard Column: | Raptor Biphenyl EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9309A0252) | ||||||||||||||||||||||||
| Temp.: | 60 °C | ||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||
| Aflatoxins standard (cat.# 34121) | |||||||||||||||||||||||||
| Ochratoxin A standard (cat.# 34122) | |||||||||||||||||||||||||
| Diluent: | 50:50 Water:methanol | ||||||||||||||||||||||||
| Conc.: | 6.25 ng/mL final concentration after sample preparation | ||||||||||||||||||||||||
| Inj. Vol.: | 5 µL | ||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||
| A: | Water, 0.05% formic acid | ||||||||||||||||||||||||
| B: | Methanol, 0.05% formic acid | ||||||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Mode: | ESI+ |
| Mode: | MRM |
| Instrument | UHPLC |
| Sample Preparation | A blended flour was prepared by mixing white rice flour (75%); brown rice flour (5%); millet flour (5%); oat flour (5%); all-purpose wheat flour (5%); and all-purpose, gluten-free flour (5%). Two grams of the flour sample were weighed into a 50-mL polypropylene centrifuge tube (cat.# 25846) and fortified at 50 µg/kg for all analytes with a stock standard solution. After sitting at room temperature for 10 minutes, 16 mL of extraction solution (80:20 acetonitrile:water) containing 0.5% formic acid were added, and the tube was stirred to create a homogenous suspension. The extraction was carried out by shaking horizontally on a digital pulse mixer (Glas-Col LLC, Terre Haute, IN) at 800 rpm for 20 minutes. After centrifuging for 5 minutes at 4000 rpm, 1 mL of extract was evaporated to dryness at 45 °C under a gentle stream of nitrogen. The dried extract was reconstituted with 1 mL of 50:50 water:methanol solution, and a 0.4 mL aliquot was transferred to and filtered using a Thomson SINGLE StEP filter vial with a 0.2 µm PTFE filter cat.# 25874. Five µL of the filtered solution was injected for the LC-MS/MS analysis. |
| Notes | The chromatogram shows peaks with the MS transition of product ion 1. Note that method development work demonstrated that whenever a new column is installed it must be rinsed and maintained under mobile phase overnight to ensure an acceptable peak shape for tenuazonic acid. Want even better performance when analyzing metal-sensitive compounds? Check out Inert LC columns at www.restek.com/inert. |
Linearity, Accuracy, and Precision
Results for linearity, accuracy, and precision are presented by commodity in Tables I-IV. Overall, the combination of a simple, universal sample preparation and analysis on a Raptor Biphenyl column under low pH conditions produced excellent results for most mycotoxins, demonstrating that the method is suitable for quantitative analysis.
To assess linearity, quadratic regression (1/x weighted) produced the best fit calibration curves for all analytes. The lowest concentration calibration standard varied due to differential MS ionization across analytes and the specific matrix effect of different food commodities. Nevertheless, most analytes were quantifiable across the full range of 0.4–400 µg/kg, and all compounds showed proper linearity with r2 >0.997 and deviations <30%.
For accuracy and precision, three batches were analyzed on three different days giving a total of nine replicates for each fortification level in each commodity. Good recoveries (72–112%) were obtained for nearly all compounds across all fortification levels and matrices, demonstrating acceptable method accuracy. Satisfactory method precision was demonstrated by the %RSDs being within 0.5–12% for all mycotoxins across all matrices.
While no incurred tenuazonic acid was detectable in peanut, its incurred concentration was relatively high in the other three food products and signal subtraction for matrix-matched calibration eliminated the low quantification level. Therefore, while no quantifiable recovery for the 5 µg/kg fortification sample was possible in wheat baby cereal, tomato puree, and blended flour, results for mid and high levels were acceptable for all sample types. This demonstrates the importance of having blank matrices for matrix-matched calibration as well as the effects of incurred amounts when they are present.
It is important to note that the use of formic acid-containing extraction solution was necessary to obtain adequate recovery for all three fumonisin B compounds, but it resulted in low recovery (24–36%) of citrinin in solid food samples. For food with high water content, such as tomato puree, acceptable recovery of all three fumonisin B compounds (90–94%) and citrinin (72–77 %) was achievable without the addition of formic acid. In addition, due to a specific matrix interference, nivalenol could not be measured in wheat baby cereal. The negative impact of matrix interference could also be observed for deoxynivalenol, fusarenon X, and patulin for tomato puree analysis in which the 5 µg/kg fortification sample was not quantifiable. Also, while isomeric 3- and 15-acetyldeoxynivalenol had distinguishable retention times, they could not be fully separated with the established LC method. Therefore, their combined recovery was calculated by integration of the 3-acetyldeoxynivalenol MS signal.
Table I: Wheat Baby Cereal Linearity, Accuracy, and Precision for Comprehensive Mycotoxin Analysis
| Linear Range | Average Percent Recovery (RSD, %) | |||||
| Concentration, µg/kg | (µg/kg) | r2 | 5 | 50 | 200 | |
| Aflatoxin B1 | 0.4 – 400 | 0.9996 | 105 (4.8) | 100 (3.0) | 79.8 (2.6) | |
| Aflatoxin B2 | 0.4 – 400 | 0.9997 | 110 (1.4) | 109 (2.8) | 106 (2.3) | |
| Aflatoxin G1 | 0.4 – 400 | 0.9999 | 105 (6.1) | 107 (1.7) | 102 (2.1) | |
| Aflatoxin G2 | 0.4 – 400 | 0.9997 | 108 (3.0) | 109 (1.3) | 104 (2.2) | |
| Ochratoxin A | 0.4 – 400 | 0.9998 | 109 (1.8) | 108 (2.1) | 94.5 (1.5) | |
| 3- + 15-Acetyldeoxynivalenol | 4.0 – 400 | 0.9994 | 104 (6.3) | 108 (1.8) | 104 (3.3) | |
| Deoxynivalenol | 2.0 – 400 | 0.9998 | 112 (4.0) | 102 (2.6) | 95.7 (1.3) | |
| Diacetoxyscirpenol | 0.8 – 400 | 0.9998 | 105 (4.0) | 107 (1.5) | 103 (1.2) | |
| Fumonisin B1 | 0.4 – 400 | 0.9999 | 94.3 (4.6) | 94.0 (2.8) | 92.3 (2.6) | |
| Fumonisin B2 | 0.4 – 400 | 0.9997 | 93.3 (4.1) | 95.1 (4.8) | 90.3 (2.9) | |
| Fumonisin B3 | 0.4 – 400 | 0.9999 | 91.8 (4.9) | 94.6 (4.9) | 91.6 (3.1) | |
| Fusarenon-X | 4.0 – 400 | 0.9971 | 99.0 (3.9) | 100 (2.9) | 103 (2.8) | |
| HT-2 | 0.4 – 400 | 0.9999 | 110 (2.4) | 111 (1.4) | 108 (1.1) | |
| Nivalenol | – | – | – | – | – | |
| T-2 | 0.4 – 400 | 0.9998 | 111 (2.1) | 110 (1.8) | 108 (2.8) | |
| α-Zearalenol | 4.0 – 400 | 0.9985 | 100 (4.9) | 102 (5.2) | 90.1 (5.8) | |
| Zearalenone | 0.8 – 400 | 0.9998 | 110 (6.7) | 110 (3.0) | 105 (3.7) | |
| Citrinin | 0.4 – 400 | 0.9996 | 26.1 (9.2) | 26.6 (3.1) | 30.1 (3.8) | |
| Patulin | 4.0 – 400 | 0.9991 | 106 (4.6) | 95.6 (5.6) | 89.2 (5.1) | |
| Alternariol | 0.4 – 400 | 0.9998 | 108 (4.1) | 108 (1.6) | 104 (1.0) | |
| Alternariol monomethylether | 0.4 – 400 | 0.9996 | 108 (4.1) | 109 (2.2) | 99.3 (2.7) | |
| Altenuene | 0.4 – 400 | 0.9999 | 110 (2.1) | 109 (2.1) | 105 (2.1) | |
| Tentoxin | 2.0 – 400 | 0.9998 | 111 (3.6) | 109 (2.5) | 103 (1.4) | |
| Tenuazonic acid | 8.0 – 400 | 0.9994 | – | 85.8 (1.7) | 87.4 (6.3) | |
| Ergocornine | 0.4 – 400 | 0.9999 | 109 (1.5) | 109 (1.4) | 102 (1.3) | |
| Ergocorninine | 0.4 – 400 | 0.9998 | 109 (3.0) | 109 (2.0) | 101 (1.9) | |
| Ergocristine | 0.4 – 400 | 0.9998 | 108 (3.1) | 108 (2.9) | 101 (4.4) | |
| Ergocristinine | 0.8 – 400 | 0.9999 | 106 (3.5) | 105 (1.4) | 101 (0.8) | |
| Ergocryptine | 0.4 – 400 | 0.9999 | 107 (2.0) | 109 (1.9) | 104 (3.4) | |
| Ergocryptinine | 0.4 – 400 | 0.9999 | 106 (1.7) | 108 (2.0) | 101 (1.1) | |
| Ergometrine | 0.4 – 400 | 0.9998 | 92.8 (7.3) | 90.0 (4.2) | 88.3 (3.6) | |
| Ergometrinine | 0.4 – 400 | 0.9997 | 101 (4.2) | 99.1 (1.9) | 94.3 (0.7) | |
| Ergosine | 0.4 – 400 | 0.9995 | 108 (2.6) | 106 (5.6) | 101 (3.2) | |
| Ergosinine | 0.4 – 400 | 0.9999 | 111 (1.8) | 109 (0.9) | 103 (1.1) | |
| Ergotamine | 0.4 – 400 | 0.9998 | 109 (1.9) | 108 (1.7) | 102 (2.8) | |
| Ergotaminine | 0.4 – 400 | 0.9999 | 109 (1.0) | 109 (0.7) | 101 (0.6) | |
Table II: Peanut Linearity, Accuracy, and Precision for Comprehensive Mycotoxin Analysis
| Linear Range | Average Percent Recovery (RSD, %) | |||||
| Concentration, µg/kg | (µg/kg) | r2 | 5 | 50 | 200 | |
| Aflatoxin B1 | 0.4 – 400 | 0.9998 | 98.2 (6.4) | 97.0 (5.2) | 89.0 (5.7) | |
| Aflatoxin B2 | 0.4 – 400 | 0.9998 | 102 (5.8) | 99.3 (4.7) | 91.3 (2.9) | |
| Aflatoxin G1 | 0.4 – 400 | 0.9997 | 98.2 (4.2) | 97.3 (3.2) | 91.2 (4.1) | |
| Aflatoxin G2 | 0.4 – 400 | 0.9998 | 104 (5.3) | 102 (3.8) | 93.5 (1.9) | |
| Ochratoxin A | 0.4 – 400 | 0.9993 | 102 (1.9) | 101 (1.1) | 97.7 (0.9) | |
| 3- + 15-Acetyldeoxynivalenol | 2.0 – 400 | 0.9997 | 101 (6.5) | 95.9 (5.8) | 91.0 (4.4) | |
| Deoxynivalenol | 4.0 – 400 | 0.9994 | 98.1 (3.5) | 93.7 (4.8) | 88.2 (3.4) | |
| Diacetoxyscirpenol | 0.4 – 400 | 0.9995 | 93.2 (4.3) | 95.4 (3.9) | 93.8 (5.0) | |
| Fumonisin B1 | 0.4 – 400 | 0.9994 | 87.2 (3.1) | 88.2 (4.5) | 87.8 (6.6) | |
| Fumonisin B2 | 0.4 – 400 | 0.9997 | 95.4 (4.7) | 92.5 (2.3) | 88.8 (3.9) | |
| Fumonisin B3 | 0.4 – 400 | 0.9997 | 90.6 (2.7) | 90.1 (3.8) | 87.7 (4.7) | |
| Fusarenon-X | 2.0 – 400 | 0.9971 | 86.9 (7.0) | 90.3 (11.0) | 88.3 (10.1) | |
| HT-2 | 0.4 – 400 | 0.9997 | 100 (2.7) | 100 (2.0) | 94.3 (3.0) | |
| Nivalenol | 8.0 – 400 | 0.9990 | – | 98.3 (6.2) | 89.0 (3.6) | |
| T-2 | 0.4 – 400 | 0.9998 | 99.1 (2.7) | 101 (1.7) | 95.9 (2.1) | |
| α-Zearalenol | 2.0 – 400 | 0.9992 | 89.2 (8.1) | 93.6 (5.5) | 94.7 (3.4) | |
| Zearalenone | 0.8 – 400 | 0.9996 | 98.3 (7.3) | 97.4 (2.8) | 91.3 (1.5) | |
| Citrinin | 0.4 – 400 | 0.9986 | 24.1 (8.7) | 25.1 (1.9) | 25.8 (3.5) | |
| Patulin | 4.0 – 400 | 0.9995 | 88.8 (12.0) | 83.6 (9.0) | 86.0 (7.2) | |
| Alternariol | 0.4 – 400 | 0.9990 | 94.2 (3.4) | 95.4 (2.4) | 96.2 (2.7) | |
| Alternariol monomethylether | 0.4 – 400 | 0.9995 | 93.5 (3.3) | 93.5 (3.7) | 89.8 (2.4) | |
| Altenuene | 0.4 – 400 | 0.9997 | 99.6 (2.0) | 99.5 (1.2) | 95.4 (1.2) | |
| Tentoxin | 0.4 – 400 | 0.9998 | 104 (2.9) | 101 (1.1) | 95.3 (1.4) | |
| Tenuazonic acid | 2.0 – 400 | 0.9997 | 92.5 (4.7) | 91.0 (2.1) | 88.5 (2.4) | |
| Ergocornine | 0.4 – 400 | 0.9998 | 93.8 (3.5) | 93.2 (4.4) | 91.2 (3.3) | |
| Ergocorninine | 0.4 – 400 | 0.9997 | 105 (3.0) | 104 (2.4) | 99.5 (3.1) | |
| Ergocristine | 0.4 – 400 | 0.9998 | 92.1 (3.8) | 91.7 (5.1) | 92.0 (2.2) | |
| Ergocristinine | 0.4 – 400 | 0.9997 | 102 (4.8) | 104 (4.3) | 102 (4.6) | |
| Ergocryptine | 0.4 – 400 | 0.9998 | 95.0 (3.0) | 94.7 (4.1) | 92.1 (1.7) | |
| Ergocryptinine | 0.4 – 400 | 0.9997 | 103 (5.3) | 105 (4.0) | 101 (4.2) | |
| Ergometrine | 0.4 – 400 | 0.9999 | 101 (2.3) | 96.2 (2.6) | 86.7 (1.9) | |
| Ergometrinine | 0.4 – 400 | 0.9999 | 93.2 (4.3) | 95.5 (1.7) | 89.1 (2.2) | |
| Ergosine | 0.4 – 400 | 0.9996 | 90.8 (2.0) | 91.8 (2.2) | 89.2 (2.6) | |
| Ergosinine | 0.4 – 400 | 0.9997 | 100 (1.1) | 102 (2.0) | 97.7 (2.2) | |
| Ergotamine | 0.4 – 400 | 0.9998 | 91.0 (2.8) | 92.6 (2.8) | 89.8 (3.6) | |
| Ergotaminine | 0.4 – 400 | 0.9997 | 98.2 (2.0) | 101 (1.5) | 96.6 (1.3) | |
Table III: Tomato Puree Linearity, Accuracy, and Precision for Comprehensive Mycotoxin Analysis
| Linear Range | Average Percent Recovery (RSD, %) | |||||
| Concentration, µg/kg | (µg/kg) | r2 | 5 | 50 | 200 | |
| Aflatoxin B1 | 0.4 – 400 | 0.9995 | 92.7 (3.8) | 97.6 (5.2) | 103 (3.0) | |
| Aflatoxin B2 | 0.4 – 400 | 0.9996 | 91.7 (4.2) | 93.3 (0.9) | 94.7 (0.4) | |
| Aflatoxin G1 | 0.4 – 400 | 0.9979 | 91.3 (1.9) | 92.2 (3.6) | 93.3 (2.5) | |
| Aflatoxin G2 | 0.4 – 400 | 0.9993 | 86.8 (8.3) | 96.4 (2.5) | 98.5 (2.5) | |
| Ochratoxin A | 0.4 – 400 | 0.9996 | 90.9 (3.5) | 93.8 (3.3) | 101 (5.9) | |
| 3- + 15-Acetyldeoxynivalenol | 4.0 – 400 | 0.9982 | 91.9 (4.3) | 98.1 (2.7) | 95.0 (1.8) | |
| Deoxynivalenol | 8.0 – 400 | 0.9991 | – | 90.3 (6.4) | 94.5 (2.6) | |
| Diacetoxyscirpenol | 0.8 – 400 | 0.9993 | 90.9 (3.8) | 94.5 (4.7) | 94.0 (1.9) | |
| Fumonisin B1 | 0.4 – 400 | 0.9999 | 91.8 (3.6) | 91.5 (1.9) | 91.9 (0.7) | |
| Fumonisin B2 | 0.4 – 400 | 0.9998 | 89.9 (4.1) | 92.9 (2.3) | 92.4 (0.8) | |
| Fumonisin B3 | 0.4 – 400 | 1.000 | 91.1 (3.6) | 93.1 (1.8) | 91.9 (0.9) | |
| Fusarenon-X | 8.0 – 400 | 0.9974 | – | 92.0 (6.8) | 94.3 (1.9) | |
| HT-2 | 0.4 – 400 | 0.9997 | 96.8 (3.1) | 96.1 (2.1) | 99.0 (1.4) | |
| Nivalenol | 20 – 400 | 0.9996 | – | 92.5 (4.5) | 93.7 (5.0) | |
| T-2 | 0.4 – 400 | 0.9992 | 92.0 (6.3) | 94.7 (1.3) | 98.6 (1.5) | |
| α-Zearalenol | 2.0 – 400 | 0.9979 | 97.7 (3.2) | 88.9 (4.2) | 90.0 (3.4) | |
| Zearalenone | 0.8 – 400 | 0.9995 | 95.0 (4.5) | 93.6 (2.2) | 95.7 (2.0) | |
| Citrinin | 0.4 – 400 | 0.9984 | 71.9 (4.7) | 76.4 (1.6) | 77.1 (1.7) | |
| Patulin | 8.0 – 400 | 0.9997 | – | 98.9 (3.6) | 103 (4.5) | |
| Alternariol | 4.0 – 400 | 0.9996 | 89.3 (4.6) | 91.8 (2.5) | 91.4 (1.3) | |
| Alternariol monomethylether | 0.4 – 400 | 0.9992 | 91.3 (6.6) | 88.7 (5.1) | 93.9 (3.9) | |
| Altenuene | 2.0 – 400 | 0.9999 | 98.4 (3.4) | 92.4 (2.1) | 92.8 (1.8) | |
| Tentoxin | 0.8 – 400 | 0.9998 | 92.5 (6.2) | 94.2 (2.2) | 95.8 (1.4) | |
| Tenuazonic acid | 8.0 – 400 | 0.9987 | – | 89.3 (4.1) | 88.5 (2.0) | |
| Ergocornine | 0.4 – 400 | 0.9998 | 91.5 (3.0) | 93.1 (1.9) | 92.9 (0.6) | |
| Ergocorninine | 0.4 – 400 | 0.9996 | 89.9 (3.8) | 92.3 (2.2) | 92.5 (3.1) | |
| Ergocristine | 0.4 – 400 | 0.9997 | 91.3 (2.9) | 94.2 (2.0) | 94.3 (0.8) | |
| Ergocristinine | 0.4 – 400 | 0.9990 | 91.6 (5.9) | 94.4 (1.8) | 95.6 (2.7) | |
| Ergocryptine | 0.4 – 400 | 0.9998 | 90.1 (3.0) | 93.5 (2.2) | 93.2 (0.7) | |
| Ergocryptinine | 0.4 – 400 | 0.9988 | 91.1 (4.3) | 95.1 (1.5) | 98.1 (1.6) | |
| Ergometrine | 0.4 – 400 | 0.9973 | 90.7 (3.6) | 88.9 (6.1) | 87.6 (3.5) | |
| Ergometrinine | 0.4 – 400 | 0.9993 | 90.6 (3.9) | 90.1 (4.4) | 89.7 (1.9) | |
| Ergosine | 0.4 – 400 | 0.9996 | 91.7 (2.2) | 90.4 (3.1) | 90.3 (1.5) | |
| Ergosinine | 0.4 – 400 | 0.9998 | 92.7 (1.4) | 93.6 (2.5) | 93.8 (0.9) | |
| Ergotamine | 0.4 – 400 | 0.9995 | 91.1 (2.2) | 90.6 (3.7) | 90.7 (1.3) | |
| Ergotaminine | 0.4 – 400 | 0.9998 | 93.6 (3.5) | 94.7 (1.7) | 94.5 (0.6) | |
Table IV: Blended Flour Linearity, Accuracy, and Precision for Comprehensive Mycotoxin Analysis
| Linear Range | Average Percent Recovery (RSD, %) | |||||
| Concentration, µg/kg | (µg/kg) | r2 | 5 | 50 | 200 | |
| Aflatoxin B1 | 0.4 – 400 | 0.9998 | 101 (2.8) | 95.5 (1.3) | 89.0 (1.5) | |
| Aflatoxin B2 | 0.4 – 400 | 1.000 | 100 (2.3) | 101 (0.9) | 88.7 (1.3) | |
| Aflatoxin G1 | 0.4 – 400 | 1.000 | 99.3 (1.7) | 100 (1.6) | 93.6 (2.2) | |
| Aflatoxin G2 | 0.4 – 400 | 0.9998 | 98.7 (3.1) | 102 (2.6) | 94.5 (2.0) | |
| Ochratoxin A | 0.4 – 400 | 1.000 | 98.1 (1.6) | 98.2 (1.3) | 82.8 (1.7) | |
| 3- + 15-Acetyldeoxynivalenol | 2.0 – 400 | 0.9998 | 98.4 (5.2) | 101 (2.9) | 100 (0.9) | |
| Deoxynivalenol | 2.0 – 400 | 0.9995 | 102 (3.5) | 97.5 (2.6) | 96.9 (0.8) | |
| Diacetoxyscirpenol | 0.4 – 400 | 0.9998 | 98.1 (6.3) | 101 (3.1) | 98.7 (1.8) | |
| Fumonisin B1 | 0.4 – 400 | 0.9999 | 100 (3.2) | 99.6 (1.7) | 96.1 (1.2) | |
| Fumonisin B2 | 0.4 – 400 | 0.9999 | 104 (2.7) | 99.6 (1.4) | 94.4 (1.6) | |
| Fumonisin B3 | 0.4 – 400 | 0.9999 | 104 (2.2) | 99.9 (1.4) | 95.9 (1.2) | |
| Fusarenon-X | 2.0 – 400 | 0.9995 | 101 (3.8) | 100 (3.7) | 98.3 (1.6) | |
| HT-2 | 0.4 – 400 | 0.9999 | 101 (1.6) | 103 (2.2) | 98.3 (1.3) | |
| Nivalenol | 8.0 – 400 | 0.9997 | – | 95.5 (4.7) | 92.9 (2.3) | |
| T-2 | 0.4 – 400 | 1.000 | 102 (1.3) | 103 (1.3) | 96.9 (1.3) | |
| α-Zearalenol | 2.0 – 400 | 0.9994 | 96.9 (3.7) | 99.0 (3.6) | 95.0 (3.3) | |
| Zearalenone | 2.0 – 400 | 0.9998 | 101 (3.8) | 102 (2.1) | 92.3 (1.4) | |
| Citrinin | 0.4 – 400 | 0.9999 | 32.3 (3.5) | 32.2 (6.3) | 35.8 (4.5) | |
| Patulin | 4.0 – 400 | 0.9993 | 93.6 (4.4) | 86.1 (3.1) | 92.2 (2.9) | |
| Alternariol | 0.4 – 400 | 0.9997 | 98.4 (2.3) | 101 (2.5) | 96.3 (3.2) | |
| Alternariol monomethylether | 0.4 – 400 | 0.9999 | 104 (2.9) | 101 (1.7) | 93.7 (1.9) | |
| Altenuene | 0.4 – 400 | 1.000 | 101 (2.9) | 101 (3.1) | 98.2 (0.5) | |
| Tentoxin | 0.4 – 400 | 0.9999 | 104 (4.2) | 105 (2.1) | 98.2 (1.9) | |
| Tenuazonic acid | 8.0 – 400 | 0.9992 | – | 92.5 (8.8) | 90.0 (9.5) | |
| Ergocornine | 0.4 – 400 | 0.9999 | 102 (2.5) | 101 (1.9) | 97.6 (1.7) | |
| Ergocorninine | 0.4 – 400 | 0.9999 | 101 (2.5) | 102 (2.6) | 95.7 (2.4) | |
| Ergocristine | 0.4 – 400 | 1.000 | 101 (1.7) | 99.8 (2.0) | 96.7 (1.8) | |
| Ergocristinine | 0.4 – 400 | 0.9999 | 102 (2.9) | 102 (3.0) | 99.3 (4.5) | |
| Ergocryptine | 0.4 – 400 | 0.9999 | 99.5 (2.7) | 99.9 (1.2) | 97.4 (1.4) | |
| Ergocryptinine | 0.4 – 400 | 1.000 | 101 (2.0) | 101 (1.8) | 95.4 (1.9) | |
| Ergometrine | 0.4 – 400 | 0.9999 | 101 (1.8) | 99.7 (3.2) | 95.3 (1.3) | |
| Ergometrinine | 0.4 – 400 | 0.9999 | 100 (3.5) | 98.5 (1.9) | 91.1 (1.9) | |
| Ergosine | 0.4 – 400 | 0.9999 | 99.9 (2.7) | 99.1 (3.0) | 98.2 (1.1) | |
| Ergosinine | 0.4 – 400 | 0.9997 | 99.2 (2.8) | 98.4 (2.8) | 97.5 (1.0) | |
| Ergotamine | 0.4 – 400 | 0.9999 | 101 (2.9) | 100 (3.1) | 96.4 (2.2) | |
| Ergotaminine | 0.4 – 400 | 0.9998 | 101 (2.3) | 99.7 (1.3) | 97.1 (1.5) | |
Conclusion
The workflow in this study provides a unique solution for mycotoxin analysis that allows simultaneous determination of Alternaria toxins and ergot alkaloid epimers along with other major mycotoxins produced by Aspergillus, Fusarium, and Penicillium fungi. The method established here combines a simple sample preparation and a fast, 11-minute analysis on a Raptor Biphenyl column under low pH conditions. Good chromatographic results were obtained—including complete separation of all six ergot alkaloids and their epimers—and the method has been demonstrated to be rugged, accurate, and precise for quantitative determination of mycotoxins in a wide variety of food products. Use of a simplified sample preparation procedure and concurrent analysis under low pH conditions provides an important opportunity for labs to improve productivity through more comprehensive methods.
Want even better performance when analyzing mycotoxins and other metal-sensitive compounds?
Learn more at www.restek.com/inert

References
- A. Tolgyesi, L. Kozma, V.K. Sharma, Determination of Alternaria toxins in sunflower oil by liquid chromatography isotope dilution tandem mass spectrometry, Molecules, 25 (2020) 1685. https://doi.org/10.3390/molecules25071685
- J. Noser, P. Schneider, M. Rother, H. Schmutz, Determination of six Alternaria toxins with UPLC-MS/MS and their occurrence in tomatoes and tomato products from the Swiss market, Mycotox Res, 27 (2011) 265-271. https://doi.org/10.1007/s12550-011-0103-x
- J.D.D. Mavungu, S.V. Malysheva, M. Sanders, D. Larionova, J. Robbens, P. Dubruel, C.V. Peteghem, S.D. Saeger, Development and validation of a new LC-MS/MS method for the simultaneous determination of six major ergot alkaloids and their corresponding epimers. Application to some food and feed commodities, Food Chemistry, 135 (2012) 292-303. https://doi.org/10.1016/j.foodchem.2012.04.098
- R. Krska, G. Stubbings, R. Macarthur, C. Crews, Simultaneous determination of six major ergot alkaloids and their epimers in cereals and foodstuffs by LC-MS-MS, Anal Bioanal Chem, 391 (2008) 563-576. https://link.springer.com/article/10.1007/s00216-008-2036-6
- D. Li, J.A. Steimling, J.D. Konschnik, S. Grossman, T. Kahler, Quantitation of mycotoxins in four food matrices comparing stable isotope dilution assay (SIDA) with matrix-matched calibration methods by LC–MS/MS, J. AOAC Int. (2019) https://doi.org/10.1093/jaoac/102.6.1673