Trifluoroacetic acid (TFA) is the perfluorinated analogue of acetic acid with the shortest possible chain length (C2) among per- and polyfluoroalkyl substances (PFAS). Together with perfluorinated C3 compounds, they are defined as ultrashort-chain PFAS. C2 and C3 PFAS are ubiquitous and very mobile in global aquatic environment including rain, snow, river, and even ocean. TFA, especially, can occur at very high concentration in both drinking and non-potable water sources. As we are developing a dilute-and-shoot LC-MS/MS method for ultrashort-chain PFAS analysis, we realized that the HPLC grade water and methanol could have TFA contamination! By applying a HILIC/ion exchange column for ultrashort-chain PFAS analysis, a detectable TFA peak was observed upon blank diluent (50:50 LC/MS grade water:HPLC methanol) injection. Further analysis showed that the LC/MS grade water contains relatively higher trace level of TFA (~5 ppt) compared to the HPLC grade methanol we regularly used for LC-MS/MS analysis. In a search for TFA-clean reagent solvents, we tested a variety of water and methanol from different vendors. Using a 10 ppt standard solution (in 50:50 DI water:EMSURE methanol) as the reference, it was shown that EMSURE methanol (with plastic container) and JT Baker methanol are much cleaner compared to other brands with either detectable or significant higher level of TFA (Figure 1). As for water reagents, the reverse osmosis (RO) and deionized (DI) waters generated in our facility are much cleaner than the LC/MS grade water.
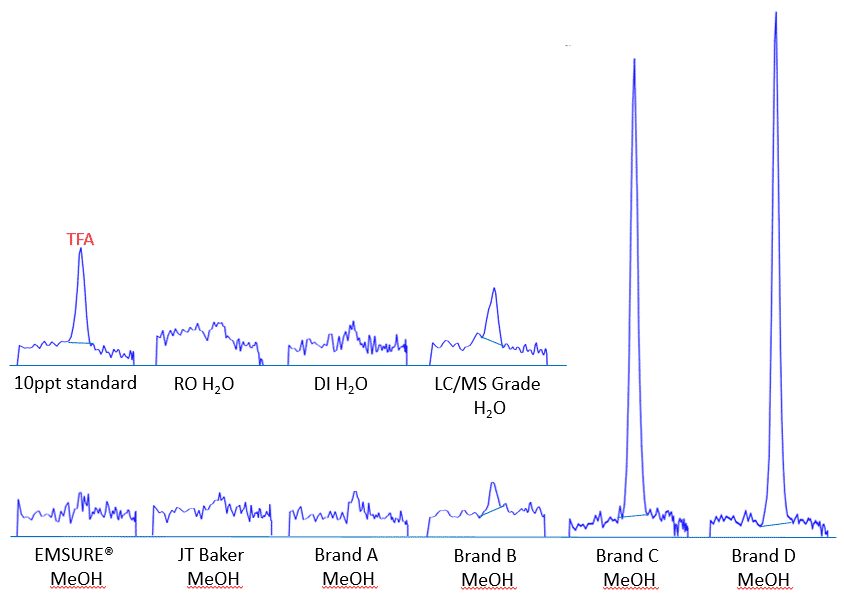
So watch out for what kinds of reagent solvents you are using if you want to include TFA in your PFAS analysis. For the trace level of TFA detection, it is necessary to use cleaner solvents for both sample preparation and LC analysis. And one more note, we also experienced that the use of glass HPLC vials could produce TFA contamination. We recommend to use polypropylene vials for ultrashort-chain PFAS or TFA LC-MS/MS analysis to avoid trace level contamination.
Are you analyzing TFA? We welcome you to share your experience in terms of what reagent solvents and labware you are using in the lab.