
When people ask you: what for work do you do? First I told them I was specialized in a separation science called “chromatography”. The question you get then often: oooh, are you guys specialized in divorces?
It’s of course logic that this is a guess, but still it’s not easy to explain what chromatography is for people with a non-physics background. As a voluntary activity I also teach music at an elementary school, and because I travel a lot, often the children, age 9-10, ask me what kind of work I do. Then I ususally tell them: “I help people in the laboratory to improve their analysis”.

Fig. 1 setup for simple test: a basket of water and a hanger to “hang” the toilet paper
This year I wanted to explain a little more to the children and also referred to the technique “chromatography”. I prepared a simple experiment that anybody can do and it takes less then 5 minutes.
In explaining chromatography, the word itself is split of 2 parts Chromos = “colors” and Graphic” = ”writing”. So chromatography is something related to writing of colors. The first scientist that discovered that was a gentlemen named Tswett. See: http://en.wikipedia.org/wiki/Mikhail_Tsvet
He discovered that some substances are containing more than one component.
A simple experiment to visualize this is described below:
Take a piece of toilet paper (one piece); Make on 1cm from the bottom little marks of a water soluble ink. I used a blue, a black and a red marker, type “stabilo sensor, from sensor technology. See fig 1.
This piece of paper was then slowly put with the bottom into water. As soon as the water touches the toilet paper on the bottom, it will be “suck-up” and it will pass the ink-spots and keep on moving up. The moment the water passes the ink-spot we see something happening. The colors start to move and change..
Fig 2 shows the result after 0.5 min, 1 min., and 2 and 4 min respectively. Zoom after 4min shown in fig 3.
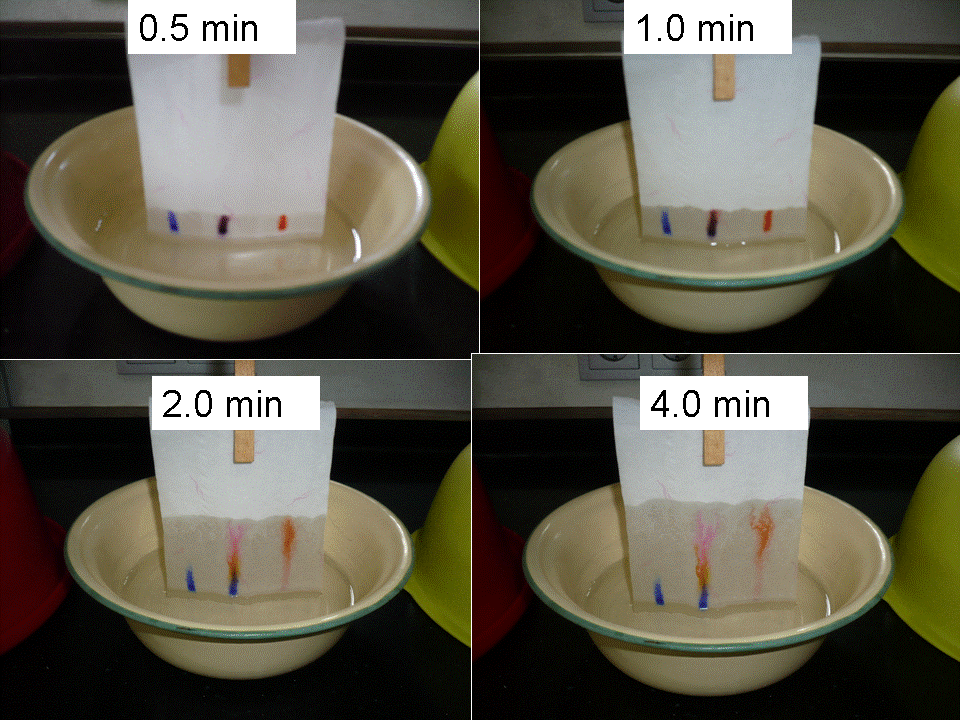
Fig. 2 result of separation of ink-colors offer 0.5, 1,2 and 4 min . Left: Blue; middle: b
Black, right: Red ink samples
We see that the “black” ink sample is now separated in 3 colors: a blue spot that is still at the bottom, a yellow spot that is in the middle and a pink one that is on top.
The red color consist of different color that even is above the pink color. The blue spot seem to stay at the same position as the blue spot in the “black” ink sample. One can do this experiment with different inks, or different types of paper or even with different types of mobile phase (alcohol, lamp-oil, etc).
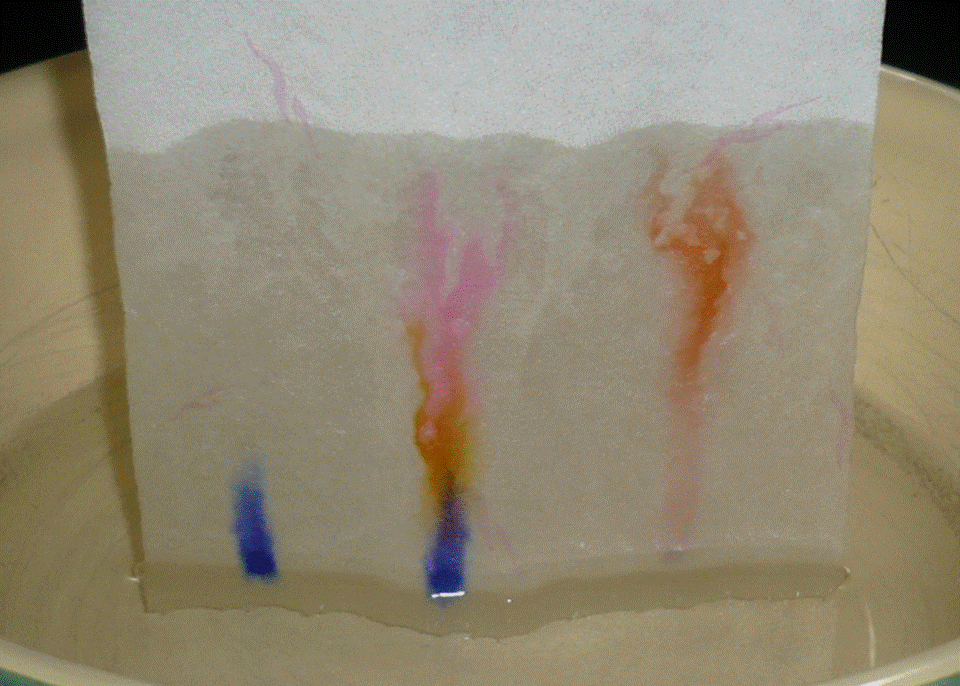
Fig. 3 detail of separation result
This is the principle of chromatography: The black-ink seem to consist of at least 3 components: a blue, a yellow and a pink one. By separating the ink one can measure the % of yellow, blue and pink that is present. Here water was used as the “mobile” phase (the moving phase) and “toilet paper” was the stationary phase (the phase that does not move). Using water (a liquid) as mobile phase we talk about “Liquid Chromatography”. Besides water one can also use “gas” as a mobile phase. In that case we talk about “Gas-Chromatography”.
I did many of these experiments wile I was doing chemistry education, and this probably triggered my interest in chromatography.
Today we can separate thousands of compounds using the technique called “ chromatography”.. it’s very powerful.


