
It must be confusing for people that see the resolution equations that are used to “explain” chromatography in GC or LC.
The challenge is that for different equations, assumptions are made, which are not listed with each equation.
Resolution, R, is dependent on 3 main parameters: The capacity factor, “k”, the theoretical plate number , “Nth”, and the selectivity factor, “alpha”.
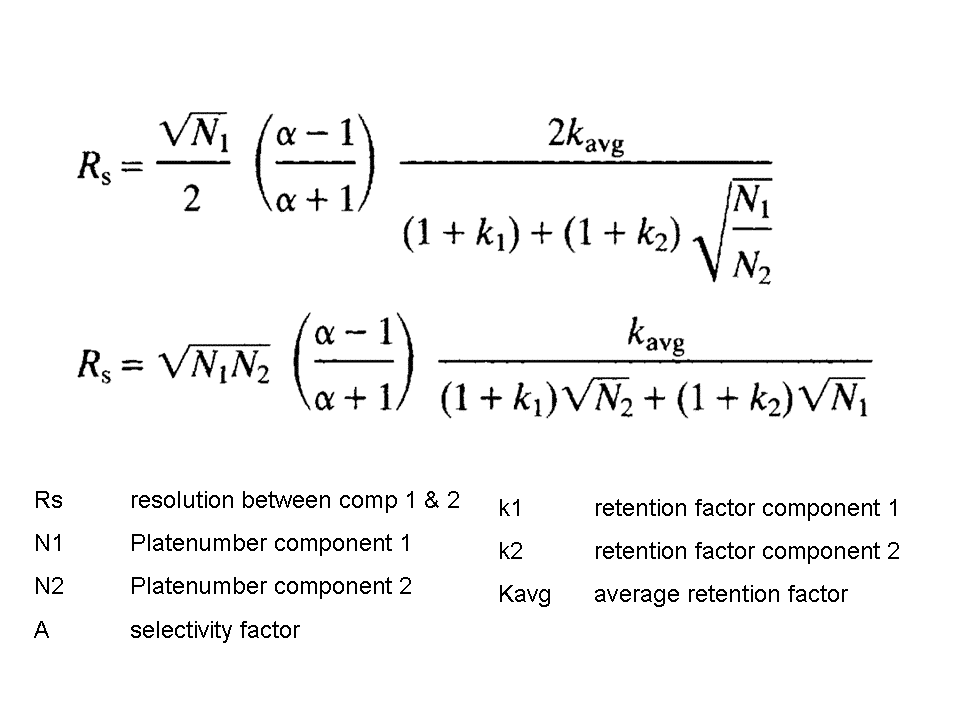
Equation 1
As R is a measure how well 2 peaks are separated, this value depends on the k and Nth for BOTH components. This makes the resolution equation very complex (equation 1). When the k of both compounds is almost the same, one can use just the “k” value. The same is possible for Nth. As the difference in k is very small, also the alpha will be small and the resolution equation can be simplified to equation 2.
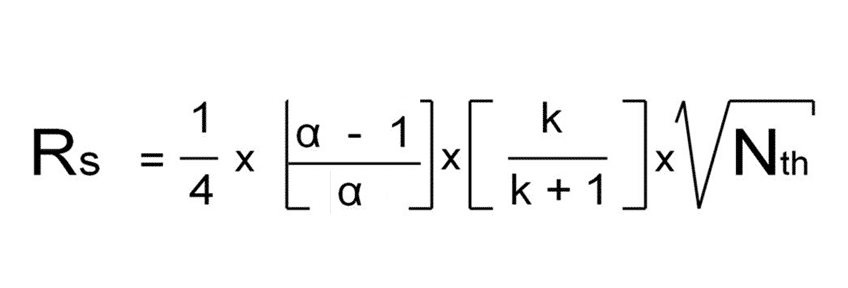
Equation 2
Equation 2 should not be used as generic anymore as its ONLY applicable for small values of alpha. If the difference in k between the 2 compounds increases the alpha also increases rapidly and according to equation 2, the “alpha-term” approaches “1”, meaning R value will not increase at higher alpha’s, which is not correct.
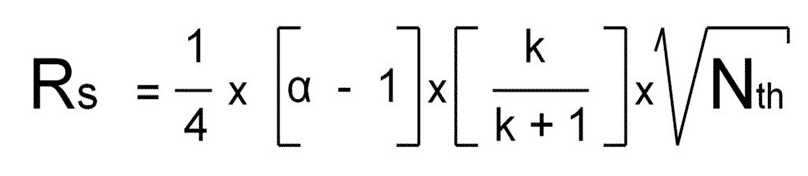
Equation 3
That’s why equation 3 is the most straightforward equation to explain the impact of all contributing parameters.
R increase always with increasing “alpha” values, independent of magnitude;
R increase also linear when small k-values are increased and fade off at higher k’s (this term becomes “1”);
R increase linear with the square root of the plate number.
For details about all equations, you can read J.P Foley, Analyst, December 1991, Vol. 116, p.1275.


