Abstract
A workflow was developed for the extraction of 12 regulated phthalates from food contact materials and plastic children’s toys for analysis by LC-MS/MS. The method utilizes a simple solvent extraction followed by solvent exchange and filtration using a Thomson filter vial. This methodology shows excellent recovery and precision, and it was used to evaluate six different sample types for percent phthalate content (w/w) to show the method’s applicability to a range of matrices.
Introduction
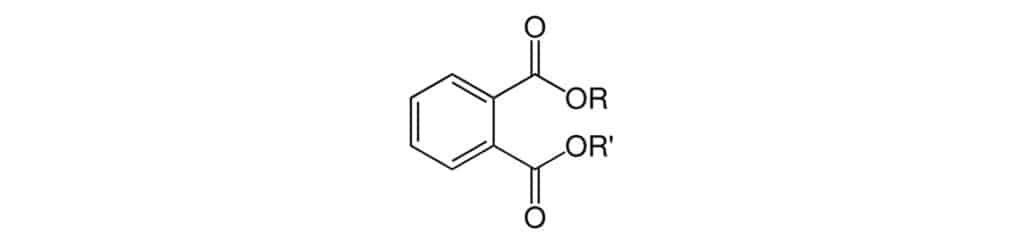
Phthalates are best known for their use as plasticizers to make polyvinyl chloride (PVC) products more flexible. They can be found in consumer goods, children’s toys, food packaging materials, cosmetics, and medical devices. Structurally, phthalates are derivatives of phthalic acid, forming phthalate esters, which all share the same precursor backbone (Figure 1). Due to their prevalence in finished goods, humans routinely come into contact with phthalates and can potentially ingest them. Phthalates have been linked to adverse health effects, such as endocrine, developmental, hepatic, renal, immunological, and reproductive disruptions [1-4]. As a precautionary measure, governing bodies throughout the world have put restrictions on the use of phthalates in children’s toys and food contact materials. Children are at a high risk of exposure to phthalates from flexible plastic toys due to their frequent hand-to-mouth behavior, which creates an easy pathway for phthalates to be ingested. Food packaging is another common route for ingestion. This can occur when phthalates migrate from food contact materials into food or drinks, and this migration can be enhanced by the commodity’s pH as well as fat, alcohol, and water content.
Although phthalates all share a similar backbone structure, the various derivatives can be associated with different health concerns, so different regulations may apply to them. Depending on the regulatory body and intended use of finished goods, several phthalates are recommended to not exceed a certain percentage of the finished product. For example, the European Union Commission Regulation Document allows dibutyl phthalic acid (DBP) to be present at up to 0.05% (w/w) and benzyl butyl phthalic acid (BBP); bis(2-ethyl-hexyl) phthalic acid (DEHP); and diisononyl phthalate (DINP) to be present at 0.1% (w/w) [5]. These values are determined from the tolerable daily intake, which is based on the effects of these compounds on the reproductive system and liver [5]. Similarly, the United States Consumer Product Safety Improvement Act (CPSIA) restricts children’s toys from having more than 0.1% (w/w) of DEHP; DBP; BBP; DINP; diisobutyl phthalate (DIBP); dipentyl phthalate (DPP/DPNP); dihexyl phthalate (DHXP); and dicyclohexyl phthalate (DCHP) [6]. In 2024, the FDA ruled that DEHP; DCHP; DINP; DIDP; diallyl phthalate (DAP); diethyl phthalate (DEP); and diisooctyl phthalate (DIOP) are authorized phthalates for food contact materials, but the agency continues to gather information on use, dietary exposure, and health information [7].
In this work, a method was developed for the LC-MS/MS analysis of 12 phthalates in food packaging and children’s toys. These phthalates cover the compounds called out in the European Union Commission Regulation Document (EUCRD) [5]; the U.S. States Consumer Product Safety Improvement Act (CPSIA) [6]; and the phthalates action plan of the U.S. Environmental Protection Agency (EPA) [8]. Phthalates can be particularly challenging to analyze due to their prevalence in plastics and equipment, which can lead to potential sample contamination from phthalates in instrumentation and lab supplies. In addition to this, several of the target analytes are also isomers sharing the same molecular weight while others share similar fragmentation patterns, making it imperative to achieve good chromatographic resolution. The method established here resolved instrument contamination peaks, accurately quantitated isomers at trace levels, and separated potentially interfering fragmenting analytes.
Experimental
Instrument Parameters
The conditions used for this LC-MS/MS method for phthalates in food packaging and toys are listed in Table I, and the analyte and internal standard MRMs are listed in Table II.
Table I: Analytical Conditions for LC-MS/MS
| Analytical Column: | Raptor Inert Biphenyl 50 x 2.1, 2.7 µm (cat.# 9309A52-T) | ||
| Guard Column: | Raptor Inert Biphenyl EXP guard column cartridge, 2.7 µm, 5 x 2.1 mm (cat#. 9309A0252-T) | ||
| Delay Column: | PFAS delay column, 5 µm, 50 x 2.1 mm HPLC column (cat.#27854) | ||
| Injection Volume: | 5 µL | ||
| Column Temperature: | 30 °C | ||
| Mobile Phase A: | Water, 0.5% acetic acid | ||
| Mobile Phase B: | Methanol | ||
| Time (min) | Flow (mL/min) | %A | %B |
| 0.00 | 0.5 | 70 | 30 |
| 1.00 | 0.5 | 70 | 30 |
| 1.01 | 0.5 | 30 | 70 |
| 4.00 | 0.5 | 25 | 75 |
| 6.00 | 0.5 | 20 | 80 |
| 7.00 | 0.5 | 15 | 85 |
| 10.00 | 0.5 | 10 | 90 |
| 11.00 | 0.5 | 0 | 100 |
| 13.00 | 0.5 | 0 | 100 |
| 13.01 | 0.5 | 70 | 30 |
| 15.00 | 0.5 | 70 | 30 |
Table II: Analyte and Internal Standard MRM Transitions
| Analyte (Abbreviation) | Precursor Ion | Product Quantifier Ion | Product Qualifier Ion | Internal Standard Group |
|---|---|---|---|---|
| Dimethyl phthalate (DMP) | 195.00 | 163.10 | 133.10 | 1 |
| Dimethyl phthlalate-d4 (DMP-4) | 198.00 | 166.00 | – | 1 |
| Diethyl phthalate (DEP) | 223.00 | 149.10 | 177.10 | 2 |
| Diethyl phthalate-d4 (DEP-d4) | 226.00 | 152.15 | – | 2 |
| Diisobutyl phthalate (DIBP) | 279.00 | 149.15 | 205.10 | 3 |
| Diisobutyl phthalate-d4 (DIBP-d4) | 283.00 | 153.05 | – | 3 |
| Dibutyl phthalate (DBP) | 279.00 | 149.05 | 205.00 | 4 |
| Dibutyl phthalate-d4 (DBP-d4) | 283.00 | 153.05 | – | 4 |
| Benzyl butyl phthalate (BBP) | 313.00 | 149.25 | 205.10 | 4 |
| Dipentyl phthalate (DPP/DPNP) | 307.00 | 149.15 | 219.00 | 5 |
| Dipentyl phthalate-d4 (DPP/DPNP-d4) | 311.00 | 153.10 | – | 5 |
| Dicyclohexyl phthalate (DCHP) | 331.00 | 167.10 | 249.05 | 6 |
| Dicyclohexyl phthalate-d4 (DCHP-d4) | 335.00 | 153.10 | – | 6 |
| Dihexyl phthalate (DHXP) | 335.00 | 149.05 | 233.00 | 7 |
| Dihexyl phthalate-d4 (DHXP-d4) | 339.00 | 153.10 | – | 7 |
| Bis-(2-ethylhexyl) phthalate (DEHP) | 391.00 | 149.05 | 167.10 | 7 |
| Diisononyl phthalate (DINP) | 419.00 | 127.00 | 275.00 | 8 |
| Di-n-octyl phthalate (DNOP) | 391.20 | 261.20 | 149.10 | 8 |
| Di-n-octyl phthalate-d4 (DNOP-d4) | 394.00 | 152.15 | – | 8 |
| Diisodecyl ortho-phthalate (DIDP) | 447.00 | 289.00 | 149.20 | 8 |
Standard Preparation
Calibrators were prepared at 5, 7.5, 10, 20, 30, 50, and 100 ng/mL; and QC standards were prepared at 8 and 75 ng/mL in 50:50 water, 0.5% acetic acid:methanol.
Sample Preparation
Six sample types were chosen to demonstrate the applicability of the methodology to a wide range of sample matrices. Three samples of plastic children’s toys (eggplant, ball, crab) and three samples of food contact materials (cling film, plastic utensil wrap, produce wrap) were obtained locally and prepared by the method described here. Each sample was prepared in triplicate and analyzed in triplicate.
Sample preparation was based on the method outlined in the Consumer Product Safety Commission Directorate for Laboratory Sciences Division of Chemistry [9]. Samples were first homogenized by cutting them into fine pieces using clean scissors. Then, 50 mg of homogenized sample was weighed into a 20 mL glass screw-thread vial (cat.# 23082). Tetrahydrofuran (THF) (5 mL) was aliquoted into the vial, which was then capped with an 18 mm magnetic screw-thread cap with a PTFE/red chlorobutyl septa (cat.# 23094). Samples were shaken for 30 minutes, which was adequate to dissolve most samples. If the sample did not dissolve after 30 minutes, it was placed on a shaker table set at 800 rpm for 2 hours. Acetonitrile (10 mL) was aliquoted into the glass vials, which were then capped and vortexed for ~30 seconds. An aliquot (2 mL) of extract was transferred to a 12 mL glass vial, and the solvent was evaporated with a gentle stream of nitrogen. The samples were then reconstituted with 400 µL of a mixture of water (0.5% acetic acid) and methanol at a ratio of 50:50; vortexed ~30 seconds; and transferred to a Thomson SINGLE StEP eXtreme 0.2 µm PTFE filter vial. Five microliters of the filtrate was injected onto the instrument.
Results and Discussion
Chromatographic Performance
Since phthalates all share the same base structure, there are several instances of isomeric derivatives. These isomers need to be chromatographically resolved for accurate quantitative analysis. In this work, two sets of isomers (diisobutyl phthalate [DIBP]/di-n-butyl phthalate [DBP] and di-n-octyl phthalate [DNOP]/bis[2-ethylhexyl] phthalate [DEHP]) were chromatographically resolved. Dimethyl phthalate (DMP) also required chromatographic resolution from a number of other compounds due to cross-analyte interference. There was also a common fragmentation of 167 m/z and 149 m/z that corresponded to protonated phthalic acid and phthalic acid anhydride, respectively. To achieve adequate resolution of these compounds, a Raptor Biphenyl column was chosen because of its ability to separate compounds based on their polarizability. The Raptor Biphenyl column showed superior resolution when compared to a C18 column.
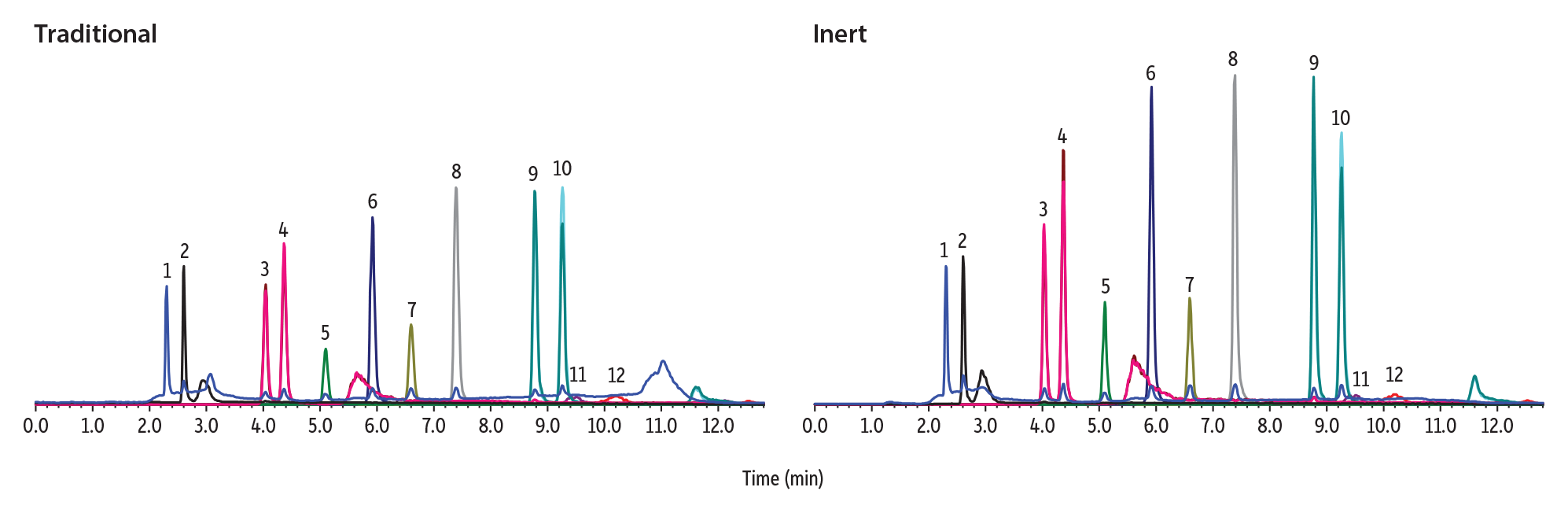
Raptor Biphenyl columns are available in both inert and traditional stainless-steel hardware. These formats were compared to determine if there was a benefit to using the inert column technology. It was discovered that when using inert LC hardware for the analysis of phthalates, peak height increased 5–76% compared to traditional LC hardware (Figure 2). Similarly, peak area was 14–59% higher when using Raptor Inert Biphenyl columns, which can provide a significant increase in sensitivity.
LC_FS0559
Peaks
| Peaks | tR (min) | Peak Area (Inert) | Peak Area (Stainless Steel) | Area % Increase | Peak Height (Inert) | Peak Height (Stainless Steel) | Height % Increase | |
|---|---|---|---|---|---|---|---|---|
| 1. | Dimethyl phthalate (DMP) | 2.31 | 19117584 | 16549407 | 16 | 5860034 | 4955690 | 18 |
| 2. | Diethyl phthalate (DEP) | 2.61 | 16492272 | 14495503 | 14 | 4702220 | 4460997 | 5 |
| 3. | Diisobutyl phthalate (DIBP) | 4.03 | 34125815 | 24265390 | 41 | 7399474 | 4540767 | 63 |
| 4. | Dibutyl phthalate (DBP) | 4.54 | 44333872 | 30394746 | 46 | 10433691 | 5916089 | 76 |
| 5. | Benzyl butyl phthalate (BBP) | 5.10 | 15407991 | 10507922 | 47 | 3030887 | 1720677 | 76 |
| 6. | Dipentyl phthalate (DPP) | 5.92 | 65583799 | 41261728 | 59 | 12955451 | 7478301 | 73 |
| 7. | Dicyclohexyl phthalate (DCHP) | 6.86 | 20403823 | 17316104 | 18 | 3496112 | 2718628 | 29 |
| 8. | Dihexyl phthalate (DHXP) | 7.38 | 71497192 | 54665430 | 31 | 13861929 | 9147576 | 52 |
| 9. | Bis(2-ethylhexyl) phthalate (DEHP) | 8.77 | 67464066 | 49840368 | 35 | 13916016 | 8943076 | 56 |
| 10. | Di-n-octyl phthalate (DNOP) | 9.26 | 14276344 | 11001910 | 30 | 2316152 | 1905245 | 22 |
| 11. | Diisononyl phthalate (DINP) | 9.41 | 4499039 | 3858257 | 17 | 260635 | 226840 | 15 |
| 12. | Diisodecyl phthalate (DIDP) | 10.21 | 3963064 | 3468306 | 14 | 220974 | 182857 | 21 |
Conditions
| Column | Raptor Inert Biphenyl (cat.# 9309A52-T) | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 50 mm x 2.1 mm ID | ||||||||||||||||||||||||||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||||||||||||||||||||||||||
| Guard Column: | Raptor Inert Biphenyl EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9309A0252-T) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Temp.: | 30 °C | ||||||||||||||||||||||||||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||||||||||||||||||||||||||
| Diluent: | 50:50 Water, 0.5% acetic acid:methanol | ||||||||||||||||||||||||||||||||||||||||||||||||
| Conc.: | 100 ng/mL | ||||||||||||||||||||||||||||||||||||||||||||||||
| Inj. Vol.: | 5 µL | ||||||||||||||||||||||||||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||||||||||||||||||||||||||
| A: | Water, 0.5% acetic acid | ||||||||||||||||||||||||||||||||||||||||||||||||
| B: | Methanol | ||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||
| Max Pressure: | 579 bar |
| Detector | Shimadzu 8060 LC-MS/MS |
|---|---|
| Ion Source: | Electrospray |
| Ion Mode: | ESI+ |
| Mode: | MRM |
| Instrument | Shimadzu Nexera X2 |
| Sample Preparation | Standards were aliquoted into 2 mL, screw-thread vials (cat.# 21143) and capped with short-cap, screw-vial closures (cat.# 24498). |
| Notes | A PFAS delay column (cat.# 27854) was installed before the injector. |
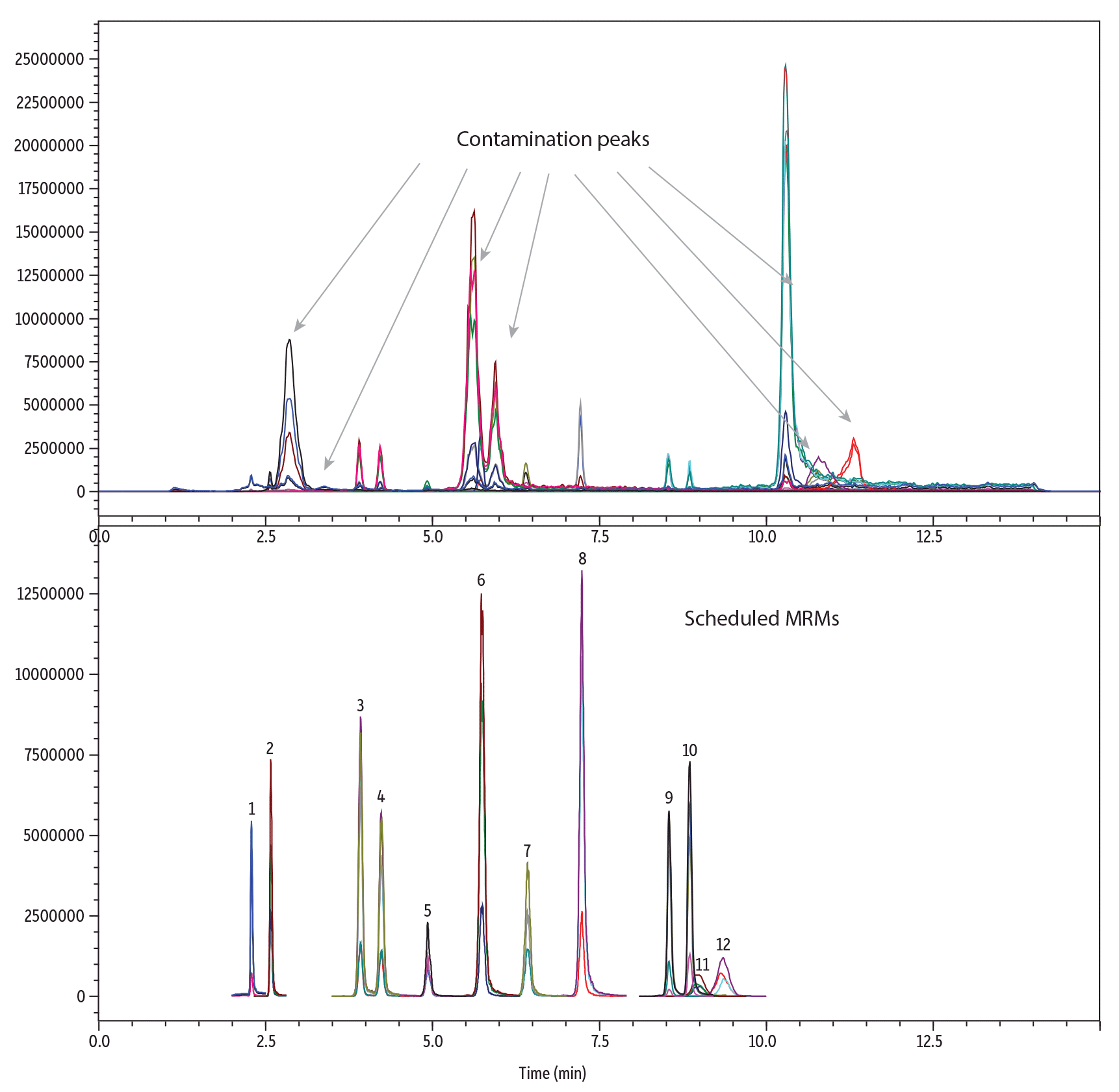
System contamination was unavoidable for the analysis of phthalates. Therefore, a PFAS delay column was installed on the instrument after the mixer and before the injector. The PFAS delay column uses hydrophobic and van der Waals interactions to delay phthalate contaminants so that they do not coelute with target analyte peaks. System contamination was observed for several analytes, and the delay column was essential for reducing baseline noise and resolving contamination peaks from analyte peaks. After the contamination peaks were resolved from the analyte peaks during method development, the MRMs were scheduled to avoid monitoring contamination peaks (Figure 3).
LC_FS0560
Peaks
| Peaks | tR (min) | Precursor Ion | Product Ion 1 | Product Ion 2 | |
|---|---|---|---|---|---|
| 1. | Dimethyl phthalate (DMP) | 2.44 | 195.00 | 163.10 | 133.10 |
| 2. | Diethyl phthalate (DEP) | 2.55 | 223.00 | 149.10 | 177.10 |
| 3. | Diisobutyl phthalate (DIBP) | 3.95 | 279.00 | 149.15 | 205.10 |
| 4. | Dibutyl phthalate (DBP) | 4.39 | 279.00 | 149.05 | 205.00 |
| 5. | Benzyl butyl phthalate (BBP) | 4.99 | 313.00 | 149.25 | 205.10 |
| 6. | Dipentyl phthalate (DPP) | 5.78 | 307.00 | 149.15 | 219.00 |
| Peaks | tR (min) | Precursor Ion | Product Ion 1 | Product Ion 2 | |
|---|---|---|---|---|---|
| 7. | Dicyclohexyl phthalate (DCHP) | 6.45 | 331.00 | 167.10 | 249.05 |
| 8. | Dihexyl phthalate (DHXP) | 7.55 | 335.00 | 149.05 | 233.00 |
| 9. | Bis(2-ethylhexyl) phthalate (DEHP) | 8.57 | 391.00 | 149.05 | 167.10 |
| 10. | Di-n-octyl phthalate (DNOP) | 8.97 | 419.00 | 127.00 | 275.00 |
| 11. | Diisononyl phthalate (DINP) | 9.01 | 391.20 | 261.20 | 149.10 |
| 12. | Diisodecyl phthalate (DIDP) | 9.48 | 447.00 | 289.00 | 149.20 |
Conditions
| Column | Raptor Inert Biphenyl (cat.# 9309A52-T) | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 50 mm x 2.1 mm ID | ||||||||||||||||||||||||||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||||||||||||||||||||||||||
| Guard Column: | Raptor Inert Biphenyl EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9309A0252-T) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Temp.: | 30 °C | ||||||||||||||||||||||||||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||||||||||||||||||||||||||
| Diluent: | 50:50 Water, 0.5% acetic acid:methanol | ||||||||||||||||||||||||||||||||||||||||||||||||
| Conc.: | 100 ng/mL | ||||||||||||||||||||||||||||||||||||||||||||||||
| Inj. Vol.: | 5 µL | ||||||||||||||||||||||||||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||||||||||||||||||||||||||
| A: | Water, 0.5% acetic acid | ||||||||||||||||||||||||||||||||||||||||||||||||
| B: | Methanol | ||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||
| Max Pressure: | 579 bar |
| Detector | Shimadzu 8060 LC-MS/MS |
|---|---|
| Ion Source: | Electrospray |
| Ion Mode: | ESI+ |
| Mode: | MRM |
| Instrument | Shimadzu Nexera X2 |
| Sample Preparation | Standards were aliquoted into 2 mL, screw-thread vials (cat.# 21143) and capped with short-cap, screw-vial closures (cat.# 24498). |
| Notes | A PFAS delay column (cat.# 27854) was installed before the injector. |
Linearity, Accuracy, and Precision
Calibration curves were constructed across a range of 5–100 ng/mL using a 1/x linear regression that showed acceptable linearity with r2 values of 0.993 or greater. This calibration range is adequate for the U.S. Product Safety Commission Directorate, the European Union Commission Regulation Document, and the EPA phthalates action plan monitoring.
The accuracy of this LC-MS/MS method for phthalates in food packaging and toys was demonstrated by assessing percent recovery for low QC (8 ng/mL) and high QC (75 ng/mL) samples, and these values fell within 14% of the nominal concentrations (86.3–104.2%). Method precision was demonstrated by %RSD values, which were ≤12.6% for all compounds. A summary of results is shown in Table III.
Table III: Results for Accuracy and Precision for QC Samples
| LQC 8 ng/mL | HQC 75 ng/mL | |||
| Phthalate | % Accuracy | %RSD | % Accuracy | %RSD |
| Dimethyl phthalate (DMP) | 98.3 | 8.8 | 91.2 | 5.9 |
| Diethyl phthalate (DEP) | 102.9 | 3.2 | 99.6 | 2.8 |
| Diisobutyl phthalate (DIBP) | 91.2 | 5.1 | 98.3 | 3.1 |
| Dibutyl phthalate (DBP) | 103.9 | 3.4 | 104.2 | 4.2 |
| Benzyl butyl phthalate (BBP) | 96.2 | 2.2 | 99.2 | 1.9 |
| Dipentyl phthalate (DPP) | 97.1 | 2.1 | 99.2 | 1.8 |
| Dicyclohexyl phthalate (DCHP) | 99.5 | 2.5 | 99.9 | 1.9 |
| Dihexyl phthalate (DHXP) | 98.6 | 2.1 | 99.7 | 1.7 |
| Bis-(2-ethylhexyl) phthalate (DEHP) | 87.6 | 7.6 | 96.3 | 5.3 |
| Diisononyl phthalate (DINP) | 86.5 | 11.1 | 96.8 | 3.1 |
| Di-n-octyl phthalate (DNOP) | 87.8 | 6.2 | 96.4 | 2.0 |
| Diisodecyl ortho-phthalate (DIDP) | 86.3 | 12.6 | 97.1 | 6.0 |
Authentic Samples
Results for real-world samples are typically reported as the percentage of phthalate (w/w) that they contain, and restrictions as low as 0.05% have been implemented for some compounds [5]. In addition, the European Union’s regulations call for four ortho-phthalates (DBP, BBP, DEHP, DIBP) to be reported as a combined number that cannot exceed 0.1% (w/w) [5]. As of July 2020, the main EU law for human health and environmental risks REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) restricted DEHP, DBP, DIBP, and BBP in swimming aids, flooring, coated fabrics, paper, recreational gear, mattresses, footwear, and office supplies [10]. This means that detection limits much lower than 0.1% (w/w) need to be achieved to accurately report the combined number to satisfy regulations. To calculate the weight percentage of phthalates in the samples, the following equations were used, and the results are presented in Table IV.
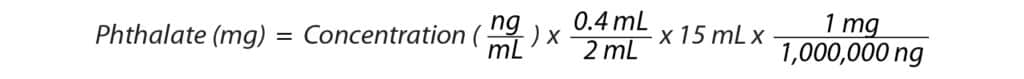
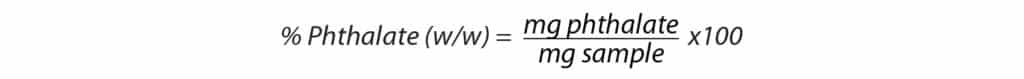
Table IV: Percent Phthalates (w/w) Detected in Real-World Samples
| Children’s Toys | Food Contact Materials | |||||
| Phthalate | Crab | Eggplant | Ball | Product Wrap | Plastic Utensil Wrap | Cling Film |
| Dimethyl phthalate (DMP) | ND | 0.000050% | 0.000430% | ND | ND | ND |
| Diethyl phthalate (DEP)* | 0.000046% | 0.000212% | 0.000064% | ND | ND | ND |
| Diisobutyl phthalate (DIBP) | 0.000247% | ND | 0.000053% | ND | ND | ND |
| Dibutyl phthalate (DBP) | 0.000195% | ND | 0.000233% | 0.000286% | ND | ND |
| Benzyl butyl phthalate (BBP) | ND | ND | ND | 0.001008% | ND | ND |
| Dipentyl phthalate (DPP) | ND | ND | ND | ND | ND | ND |
| Dicyclohexyl phthalate (DCHP) | ND | ND | ND | ND | ND | ND |
| Dihexyl phthalate (DHXP) | ND | ND | ND | ND | ND | ND |
| Bis-(2-ethylhexyl) phthalate (DEHP)* | ND | 0.000753% | ND | ND | 0.000281% | ND |
| Diisononyl phthalate (DINP)* | ND | ND | ND | ND | ND | ND |
| Di-n-octyl phthalate (DNOP) | ND | ND | ND | ND | ND | ND |
| Diisodecyl ortho-phthalate (DIDP) | ND | ND | ND | ND | ND | ND |
| EU Ortho Phthalates (sum DBP, BBP, DEHP, DIDP) | 0.000195% | 0.000753% | 0.000233% | 0.001294% | 0.000281% | ND |
*Incurred levels were present in the blank samples for these analytes and were subtracted from the fortified samples.
Column Reproducibility
Column reproducibility was tested using three different lots of Raptor Inert Biphenyl columns, and excellent retention time stability was achieved as demonstrated by <0.55% retention time difference across the lots (Table V).
Table V: Retention times were stable across three lots of analytical columns, indicating that the performance of Raptor Inert Biphenyl columns is highly reproducible.
| Phthalate | Lot 1 Retention Time (min) | Lot 2 Retention Time (min) | Lot 3 Retention Time (min) | ± % Difference |
|---|---|---|---|---|
| Dimethyl phthalate (DMP) | 2.32 | 2.32 | 2.31 | 0.09% |
| Diethyl phthalate (DEP) | 2.65 | 2.64 | 2.61 | 0.20% |
| Diisobutyl phthalate (DIBP) | 4.19 | 4.15 | 4.03 | 0.50% |
| Dibutyl phthalate (DBP) | 4.57 | 4.51 | 4.37 | 0.55% |
| Benzyl butyl phthalate (BBP) | 5.33 | 5.29 | 5.10 | 0.55% |
| Dipentyl phthalate (DPP) | 6.15 | 6.10 | 5.92 | 0.48% |
| Dicyclohexyl phthalate (DCHP) | 6.88 | 6.82 | 6.59 | 0.53% |
| Dihexyl phthalate (DHXP) | 7.58 | 7.54 | 7.38 | 0.33% |
| Bis-(2-ethylhexyl) phthalate (DEHP) | 8.98 | 8.92 | 8.77 | 0.30% |
| Diisononyl phthalate (DINP) | 9.70 | 9.65 | 9.51 | 0.25% |
| Di-n-octyl phthalate (DNOP) | 9.47 | 9.40 | 9.26 | 0.28% |
| Diisodecyl ortho-phthalate (DIDP) | 10.42 | 10.42 | 10.29 | 0.16% |
Method Robustness
The robustness of the method was tested by fortifying the plastic ball extract with phthalates at 20 ppb. One of the most challenging separations is that of diethyl phthalate (DEP), since it needs to be resolved from both dimethyl phthalate (DMP) and a DEP contamination peak. The fortified sample extract was injected onto the instrument repeatedly for 150 injections, and the resolution between DEP, DMP, and the interference DEP peak was assessed. The resolution achieved for DEP did not change, even after subsequent injections, demonstrating that the method was robust.
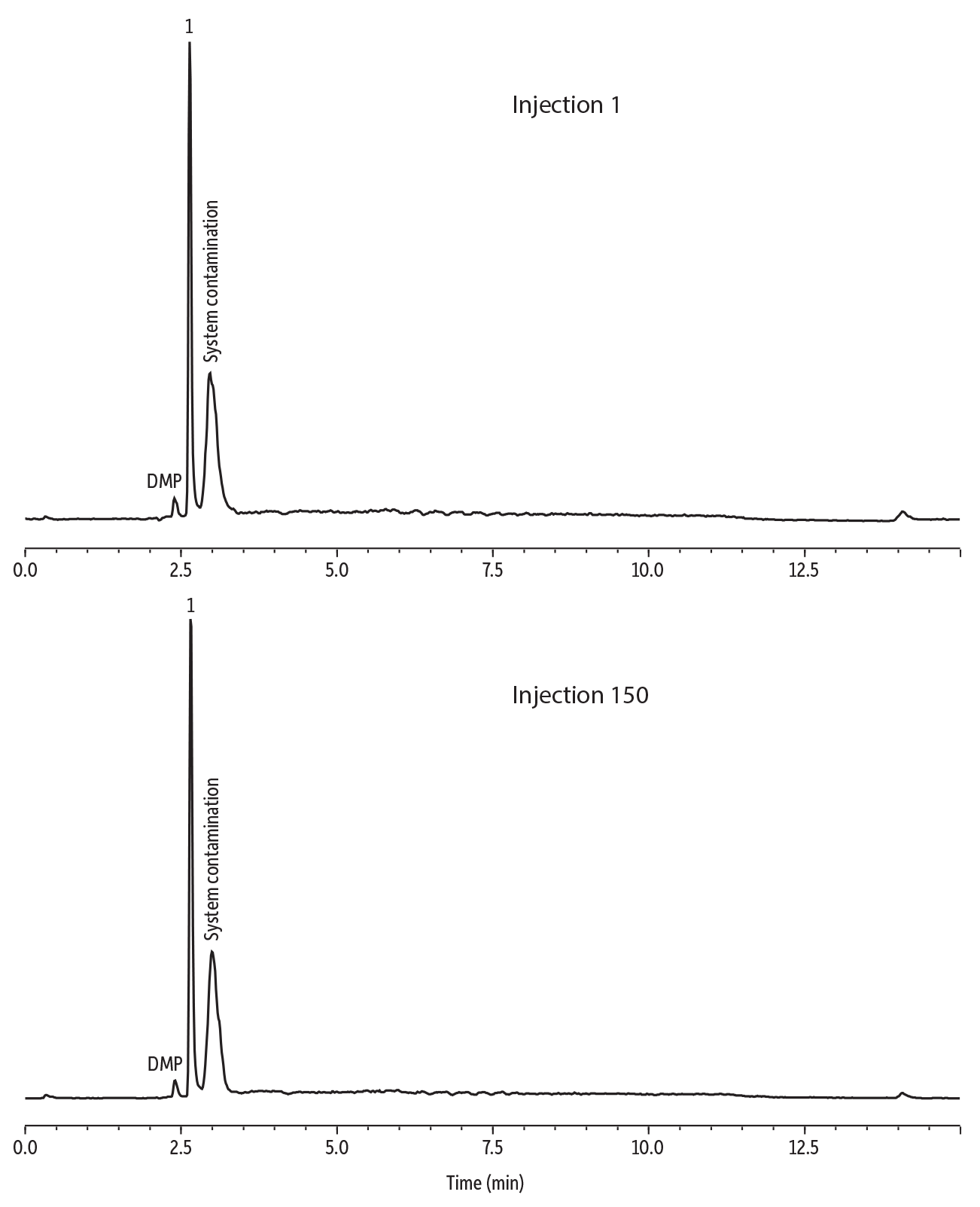
LC_FS0562
Peaks
| Peaks | tR (min) | Precursor Ion | Product Ion 1 | |
|---|---|---|---|---|
| 1. | Diethyl phthalate (DEP) | 2.64 | 223.00 | 149.10 |
Conditions
| Column | Raptor Inert Biphenyl (cat.# 9309A52-T) | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 50 mm x 2.1 mm ID | ||||||||||||||||||||||||||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||||||||||||||||||||||||||
| Guard Column: | Raptor Inert Biphenyl EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9309A0252-T) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Temp.: | 30 °C | ||||||||||||||||||||||||||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||||||||||||||||||||||||||
| Diluent: | 50:50 Water, 0.5% acetic acid:methanol | ||||||||||||||||||||||||||||||||||||||||||||||||
| Conc.: | 20 ng/mL | ||||||||||||||||||||||||||||||||||||||||||||||||
| Inj. Vol.: | 5 µL | ||||||||||||||||||||||||||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||||||||||||||||||||||||||
| A: | Water, 0.5% acetic acid | ||||||||||||||||||||||||||||||||||||||||||||||||
| B: | Methanol | ||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||
| Max Pressure: | 579 bar |
| Detector | Shimadzu 8060 LC-MS/MS |
|---|---|
| Ion Source: | Electrospray |
| Ion Mode: | ESI+ |
| Mode: | MRM |
| Instrument | Shimadzu Nexera X2 |
| Sample Preparation | Homogenized sample (50 mg) was weighed into a clean 20 mL glass screw-thread vial (cat.# 23082). Tetrahydrofuran (THF) (5 mL) was aliquoted into the vial and capped with an 18 mm magnetic screw-thread cap with a PTFE/red chlorobutyl septum (cat.# 23094). Samples were then shaken for 30 minutes for samples that fully dissolved or 2 hours for ones that did not using a shaker table at 800 rpm. Acetonitrile (10 mL) was aliquoted into the glass vials, capped, and vortexed for ~30 seconds. An aliquot (2 mL) was transferred to a 12 mL glass vial and the solvent dried off with a gentle stream of nitrogen. The samples were then reconstituted with a mixture of 50:50 water, 0.5% acetic acid:methanol, fortified with phthalates at a concentration of 20 ng/mL, vortexed ~30 seconds, and transferred to a Thomson SINGLE StEP eXtreme 0.2 µm PTFE filter vial and 5 µL was injected onto the instrument. |
| Notes | A PFAS delay column (cat.# 27854) was installed before the injector. |
Conclusion
The method outlined in this work addresses many of the issues associated with analyzing phthalates in food contact materials and children’s toys. In order to achieve adequate sensitivity and selectivity, a Raptor Inert Biphenyl column was utilized to resolve isomers and compounds that are susceptible to cross-analyte interference due to similar degradation pathways and product ions. A delay column was inserted before the injector to move contamination peaks away from the target analyte peaks, allowing for accurate quantitation. The sample preparation uses a simple solvent extraction technique that is quick and easy and amenable to high-throughput labs.
References
- Agency for Toxic Substances and Disease Registry, Di(2-ethylhexyl)phthalate (DEHP)-ToxFAQs, CDC. https://www.atsdr.cdc.gov/toxfaqs/tfacts9.pdf, 2022 (accessed February 17, 2025).
- Agency for Toxic Substances and Disease Registry, Toxicological profile for di-n-butyl phthalate, CDC. https://www.atsdr.cdc.gov/ToxProfiles/tp135.pdf (accessed February 17, 2025).
- Agency for Toxic Substances and Disease Registry, Toxicological profile for di-n-octylphthalate (DNOP), CDC. https://www.atsdr.cdc.gov/toxprofiles/tp95.pdf (accessed February 17, 2025).
- Agency for Toxic Substances and Disease Registry, Toxicological profile for diethyl phthalate, CDC. https://www.atsdr.cdc.gov/ToxProfiles/tp73.pdf (accessed February 17, 2025).
- EUR-lex, Commission Regulation (EU) 2023/1442 of 11 July 2023 amending Annex I to Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food, as regards changes to substance authorisations and addition of new substances (text with EEA relevance), 2023. https://eur-lex.europa.eu/eli/reg/2023/1442/oj
- United States Consumer Product Safety Commission, Phthalates business guidance, 2025. https://www.cpsc.gov/Business–Manufacturing/Business-Education/Business-Guidance/Phthalates
- U.S. Food and Drug Administration, Phthalates in food and packaging and food contact applications, 2024. https://www.fda.gov/food/food-additives-and-gras-ingredients-information-consumers/phthalates-food-packaging-and-food-contact-applications
- U.S. Environmental Protection Agency, Phthalates action plan, 2012. https://19january2017snapshot.epa.gov/sites/production/files/2015-09/documents/phthalates_actionplan_revised_2012-03-14.pdf