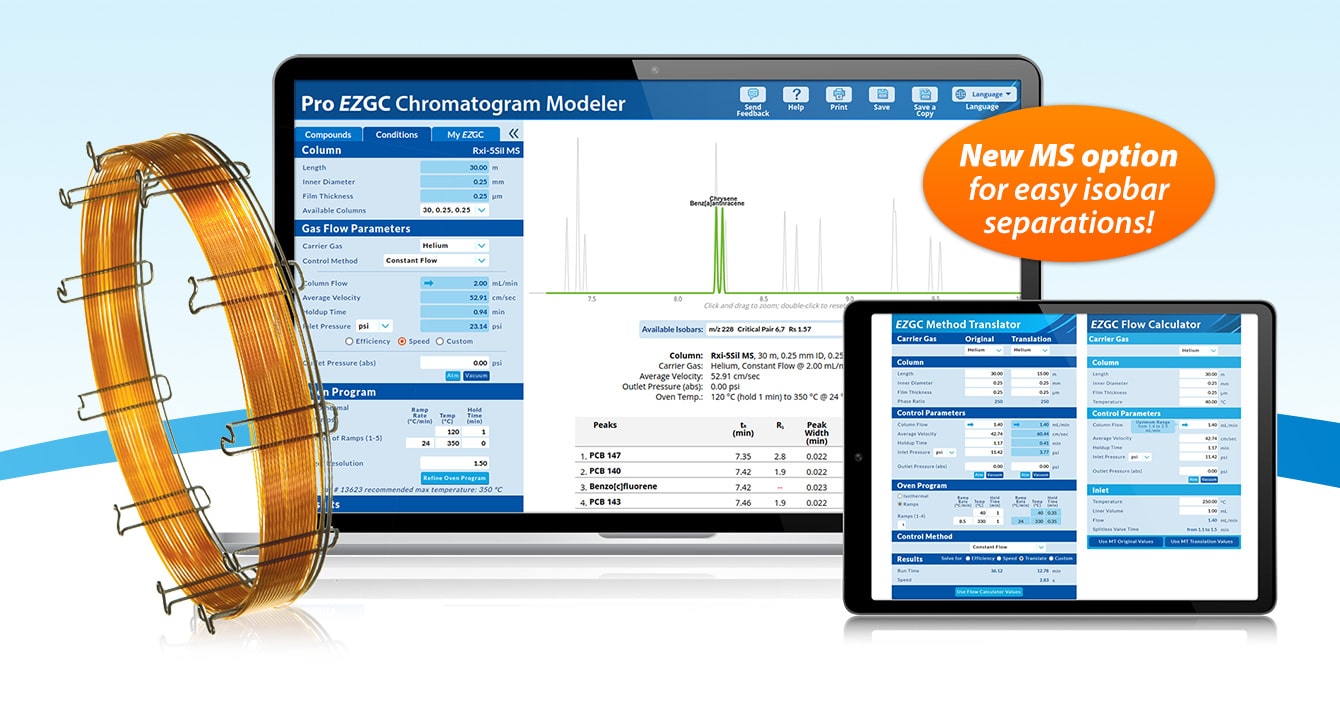
突破GC-MS半挥发性有机物痕量分析检测限的技术边界
19 Jan 2026

Authors
-

Jessi is an advanced scientist in the GC applications group where her work focuses on environmental and food contaminants. Prior to joining Restek in 2023, she worked for the Mississippi State Chemical Lab as a pesticide residue analyst, primarily testing regulatory samples, agricultural samples, environmental samples, foods, and other consumer products via GC-MS, GC-MS/MS, GC-FID, and GC-ECD. Jessi holds a BS in chemistry and a BA in Mandarin Chinese from Mississippi State University.
View all posts -

Erica is an interdisciplinary GC applications and technologies scientist at Restek. She obtained her bachelor's degree in forensic biology from The Pennsylvania State University, and her doctorate from Virginia Tech in plant pathology, physiology, and weed science. Since joining Restek in 2021, she has worked with a wide variety of GC columns, including fused silica, MXT, PLOT, and packed columns as well as accessories, such as liners, valves, and methanizers.
View all posts -

Dr. Ramkumar Dhandapani is a seasoned analytical chemist with over 23 years of experience in the chromatography industry and a Ph.D. in analytical chemistry. During his career, he has specialized in method development, validation, and the troubleshooting of chromatography methods. He has developed numerous regulatory-compliant methods across diverse sectors, including environmental analysis, food quality and safety, pharmaceutical, fuels, and chemical industries. Currently, Dr. Dhandapani is the Director of Product Management at Restek, he is keen on innovation in chromatography and scaling breakthrough innovations to market as commercial products.
View all posts -

Colton Myers is the R&D manager for sample preparation at Restek Corporation with over 10 years of experience in product development and application innovation, particularly in solid phase microextraction (SPME) and volatile analysis. He has made contributions across various industries, authoring multiple peer-reviewed publications. Starting his career in quality control before transitioning to the GC Innovations team, Colton now leads a team dedicated to advancing sample preparation and collection technologies. He holds a BS in chemistry from Juniata College.
View all posts